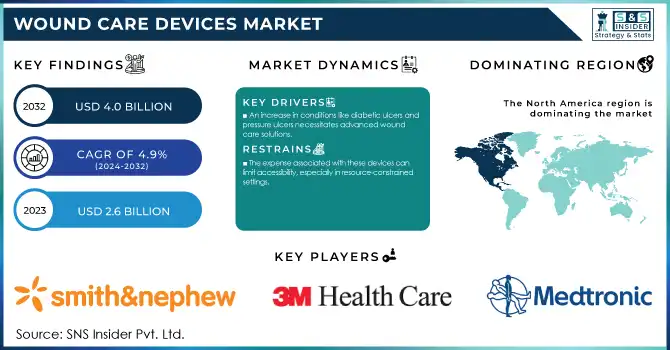
The Wound Care Devices Market Size was valued at USD 2.6 Billion in 2023 and is expected to reach USD 4.0 Billion by 2032, and grow at a CAGR of 4.9% over the forecast period 2024-2032.
To Get More Information on Wound Care Device Market - Request Sample Report
The global wound care devices market is booming, driven by various growth factors. Chronic wounds like diabetic foot ulcers, pressure ulcers and venous leg ulcers are the greatest contributing factors. Data from the Centers for Disease Control and Prevention (CDC) estimates that, in 2023, 37.3 million Americans (11.3% of the population) have diabetes, and 96 million adults (38.0%) have prediabetes. Such a high incidence of diabetes leads to a greater number of diabetic foot ulcers, a key market application for wound care devices. Moreover, the rising geriatric population is supporting the market growth, as older people are more vulnerable to chronic wounds. According to the U.S. Census Bureau, all baby boomers will be over the age of 65 by 2030, increasing the number of older Americans to 73 million. The growing geriatric population is anticipated to raise the need for advanced wound care.
Moreover, innovations in wound care devices such as smart dressings, negative pressure wound therapy systems, and others are improving treatment efficiency, which will boost market growth. The demand for the market has also been indirectly affected by the COVID-19 pandemic, as hospital and home care settings put more emphasis on healthcare and wound care than ever before. Market growth is also augmented by government efforts to enhance healthcare infrastructure and minimize economic insurance of chronic wounds. For example, the U.S. Department of Health and Human Services has initiated efforts to prevent and treat diabetic foot ulcers because of their large cost to public health and the healthcare systems.
Drivers
An increase in conditions like diabetic ulcers and pressure ulcers necessitates advanced wound care solutions.
Innovations such as negative pressure wound therapy (NPWT) systems and bioactive materials enhance treatment efficacy and patient outcomes.
Innovation in the field of sciences or technology is also one of the leading factors contributing to the wound care devices market growth. One such advance is a biodegradable suture that provides electrical stimulation via patient motion, promoting faster wound healing and smaller infection risk. When the suture moves through the skin, the process works through the triboelectric effect, creating an electric field surrounding the wound for faster healing.
Also, with the advent of smart wound care devices, like electronic dressings with sensors, it is now possible to monitor the condition of the winding in real time. They are also capable of detecting the presence of bacteria or an overabundance of fluid, as well as dispensing targeted treatments where needed, ultimately speeding up the recovery process while also reducing the need for follow-up interventions. Not only do these technological innovations enhance the efficiency of wound management, but they are also cost-effective solutions that make advanced wound care more accessible to a wider patient population.
Restraints
The expense associated with these devices can limit accessibility, especially in resource-constrained settings.
Complex approval processes can delay the introduction of new products to the market.
The high cost of advanced technologies such as negative pressure wound therapy (NPWT) systems, bioengineered skin substitutes, and advanced dressings is one of the major restraints in the wound care devices market. These solutions are typically made from advanced materials and require complicated manufacturing processes, driving up their overall cost. While they offer superior outcomes in managing chronic wounds and promoting faster healing, their pricing can be prohibitive for many patients, particularly in low-income or resource-constrained settings. In addition, these high-end devices are not user-friendly enough to be integrated with routine care in developing parts of the world, as the healthcare system has a limited budget. Even in developed markets, insurance coverage often varies, with some payers limiting reimbursements for high-cost wound care products. The cost can hinder healthcare providers from adopting the system, to change patients still rely on traditional methods that may not be able to provide the same effectiveness.
By Product Type
In 2023, negative pressure wound therapy (NPWT) held the largest share 85% of the market. The reasons for this dominance are related to the very strong efficacy in the treatment of complex and chronic wound management but they also promote faster healing and reduce the wound infection. The Centers for Medicare & Medicaid Services (CMS) has recognized the clinical benefits of NPWT and provides coverage for its use in various wound types, which has significantly contributed to its widespread adoption. AHRQ 2021 published a report suggesting that there is emerging evidence to support the use of NPWT for several types of wounds, including diabetic foot ulcers, and pressure ulcers. The Food and Drug Administration (FDA) of the United States has also approved many NPWT devices, which confirms their safety and effectiveness.
Additionally, the National Institutes of Health (NIH) has funded numerous studies on NPWT, contributing to the growing body of evidence supporting its use. The portability and ease of use of modern NPWT devices has also expanded the scope of their utilization in hospital and home care settings, propelling the market growth. Also, the cost-effectiveness of NPWT against standard wound management techniques has rendered NPWT a preferable option for healthcare providers and payers given that it may reduce overall treatment costs and length of hospital stay.
By End User
In 2023, the largest revenue share of the market was held by the hospitals segment. There are a number of reasons for this leadership position, including the number of complex wound cases being treated in hospitals, as well as access to advanced wound care technologies in the hospital environment. In 2023, there are 6,093 hospitals (source American Hospital Association (AHA)) in the USA alone, creating an immense ecosystem for usage of wound care devices. According to the Centers for Disease Control and Prevention (CDC), in 2019, there were 36.2 million hospital admissions in the U.S., and countless of those admissions included patients needing wound care. Severe and chronic wounds are usually treated in hospitals, as they require an environment with infrastructure, qualified professionals, and modern equipment to manage complex cases. Alongside the Centers for Medicare & Medicaid Services (CMS) efforts that establish quality measures and reimbursement policies to encourage hospitals to enhance patient outcome systems to reduce hospital-acquired pressure ulcer, it can lead to the wider use of advanced wound care technologies in hospitals. Thereby, propelling the demand for advanced wound care management products in the hospitals. Moreover, the NPUAP has also set the standards for pressure ulcer prevention and management which are commonly adopted by hospitals, making use of specialized wound care devices.
By Indication
Diabetic foot ulcers segment lead the market in 2023. The increasing number of people with diabetes and its complications around the globe is the reason behind its prominence. Historically, the Centers for Disease Control and Prevention (CDC) estimated that in 2020, approximately 34.2 million Americans (10.5% of the population) had diabetes; 1 out of 4 survived long enough to develop a diabetic foot ulcer (DFU) in their lifetime. Diabetic foot ulcers are the most common cause of non-traumatic lower extremity amputations, according to the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). These statistics indicate the high unmet need for effective wound care devices in the diabetic foot ulcer market. The economic impact of diabetic foot ulcers has been great, with the American Diabetes Association estimating that total direct medical costs of diabetes per year in the U.S. reached $237 billion in 2017, a large part of which was accounted for by foot complications. This financial burden has led to more investments in advanced wound care technologies for diabetic foot ulcers. Furthermore, the U.S. Department of Health and Human Services' Healthy People 2020 initiative included objectives to reduce the rate of lower extremity amputations in persons with diagnosed diabetes, which has spurred the development and adoption of innovative wound care devices for diabetic foot ulcers.
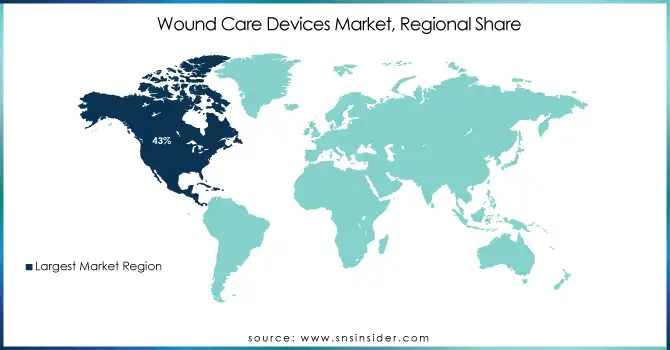
In 2023 North America accounted for the largest share of the wound care devices market. In 2023, the North American market share is estimated to be about 43% of the global market. Several factors across the region such as developed healthcare infrastructure, high healthcare expenditure, and high patient population with chronic wounds contribute to its dominance. As the prevalence of chronic wounds increases with age, the U.S. Census Bureau projects that the number of Americans aged 65 years and older will almost double in those years, from 52 million in 2018 to 95 million by 2060.
The fast growth of the Asia-Pacific region is attributed to the improvement of healthcare infrastructure, rising awareness of advanced wound care, along the surge in the diabetic population. According to the International Diabetes Federation (IDF), 163 million adults were living with diabetes in the Western Pacific region (including much of Asia) in 2019, and the number of victims is expected to rise to 212 million by 2045. Such an increase in the incidence of diabetes will propel the demand for wound care devices, especially for diabetic foot ulcers. The increasing investment in healthcare access and quality from governments in countries such as China and India is driven by the need for sustainable large market growth.
Do You Need any Customization Research on Wound Care Device Market - Enquire Now
Key Service Providers/Manufacturers
Smith & Nephew (PICO 7, Allevyn Gentle Border)
3M Healthcare (Tegaderm, Coban 2)
ConvaTec Group plc (Avelle, Aquacel Ag+)
Medtronic (V.A.C. Therapy, Kendall SCD)
Molnlycke Health Care AB (Mepilex, Mepitel One)
Coloplast A/S (Biatain Silicone, SenSura Mio)
Hollister Incorporated (Endoform Dermal Template, Hydrofera Blue READY)
Cardinal Health (Kendall Foam Dressings, CURAD Non-Adherent Pads)
Derma Sciences, Inc. (Integra LifeSciences) (MEDIHONEY, TCC-EZ)
B. Braun Melsungen AG (Prontosan, Askina Calgitrol Ag)
Hartmann Group (HydroClean, HydroTac)
Kinetic Concepts, Inc. (Acelity) (ActiV.A.C., Prevena Therapy)
Medline Industries, Inc. (SilvaSorb Gel, Optifoam Gentle)
Advanced Medical Solutions Group plc (LiquiBand, ActivHeal Foam)
Integra LifeSciences Holdings Corporation (Omnigraft, PriMatrix Dermal Repair Scaffold)
Lohmann & Rauscher (L&R) (Suprasorb, Debrisoft)
Zimmer Biomet (Gel-One, wound VAC systems)
MiMedx Group, Inc. (EpiFix, AmnioFix)
Organogenesis Inc. (PuraPly, Affinity)
Convexity Scientific (AquaMed Advanced, PICO technology solutions)
Recent Developments
Smith & Nephew launched its new innovative negative pressure wound therapy system which has received clearance from the FDA for hospital and homecare use in June 2024. It uses AI-powered pressure adjustment technology for the quick healing process of wounds.
In March 2025, smart wound dressing was launched by Medtronic where sensors are integrated for wound healing and data are transferred to care providers as real-time updates. The product aims to revolutionize wound care management by enabling early intervention and personalized treatment plans.
In November 2024, 3M Healthcare launched novel antimicrobial wound dressings incorporating silver nanoparticles to treat chronic wounds infected by antibiotic-resistant bacteria. CDC's efforts to combat the rising threat of antibiotic resistance in healthcare settings are reflected in this product launch.
| Report Attributes | Details |
| Market Size in 2023 | US$ 2.6 Bn |
| Market Size by 2032 | US$ 4.0 Bn |
| CAGR | CAGR of 4.9% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments |
|
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]). Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia Rest of Latin America) |
| Company Profiles |
Smith & Nephew, 3M Healthcare, ConvaTec Group plc, Medtronic, Molnlycke Health Care AB, Coloplast A/S, Hollister Incorporated, Cardinal Health, Derma Sciences, Inc. (Integra LifeSciences), B. Braun Melsungen AG, Hartmann Group, Kinetic Concepts, Inc. (Acelity), Medline Industries, Inc., Advanced Medical Solutions Group plc, Integra LifeSciences Holdings Corporation, Lohmann & Rauscher (L&R), Zimmer Biomet, MiMedx Group, Inc., Organogenesis Inc., Convexity Scientific. |
| Key Drivers |
|
| Market Restraints |
|
Ans. For the projection period, the compound annual growth rate for the Wound Care Devices Market is 4.7%.
Ans. By 2031, the market for Bovine Mastitis is expected to be worth USD 3.78 Billion.
Ans. The Therapy Devices category is the fastest growing in the Wound Care Devices Market.
Ans. In 2023, North America will have the largest share.
Ans. The primary trend in the wound management devices market is an increase in the global elderly population, which promotes market growth.
Table of Contents
1. Introduction
1.1 Market Definition
1.2 Scope (Inclusion and Exclusions)
1.3 Research Assumptions
2. Executive Summary
2.1 Market Overview
2.2 Regional Synopsis
2.3 Competitive Summary
3. Research Methodology
3.1 Top-Down Approach
3.2 Bottom-up Approach
3.3. Data Validation
3.4 Primary Interviews
4. Market Dynamics Impact Analysis
4.1 Market Driving Factors Analysis
4.1.1 Drivers
4.1.2 Restraints
4.1.3 Opportunities
4.1.4 Challenges
4.2 PESTLE Analysis
4.3 Porter’s Five Forces Model
5. Statistical Insights and Trends Reporting
5.1 Incidence and Prevalence (2023)
5.2 Prescription Trends (2023), by Region
5.3 Device Volume, by Region (2020–2032)
5.4 Healthcare Spending, by Region (2023)
5.5 Feature Analysis, by Device Type
6. Competitive Landscape
6.1 List of Major Companies, By Region
6.2 Market Share Analysis, By Region
6.3 Product Benchmarking
6.3.1 Product specifications and features
6.3.2 Pricing
6.4 Strategic Initiatives
6.4.1 Marketing and promotional activities
6.4.2 Distribution and Supply Chain Strategies
6.4.3 Expansion plans and new product launches
6.4.4 Strategic partnerships and collaborations
6.5 Technological Advancements
6.6 Market Positioning and Branding
7. Wound Care Devices Market Segmentation, By Product Type
7.1 Chapter Overview
7.2 Negative pressure Wound Therapy (NPWT)
7.2.1 Negative pressure Wound Therapy (NPWT) Market Trends Analysis (2020-2032)
7.2.2 Negative pressure Wound Therapy (NPWT) Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3 Extracorporeal Shock Wave Therapy (ESWT)
7.3.1 Extracorporeal Shock Wave Therapy (ESWT) Market Trends Analysis (2020-2032)
7.3.2 Extracorporeal Shock Wave Therapy (ESWT) Market Size Estimates and Forecasts to 2032 (USD Billion)
7.4 Hyperbaric Oxygen Therapy (HBOT)
7.4.1 Hyperbaric Oxygen Therapy (HBOT) Market Trends Analysis (2020-2032)
7.4.2 Hyperbaric Oxygen Therapy (HBOT) Market Size Estimates and Forecasts to 2032 (USD Billion)
7.5 Others
7.5.1 Others Market Trends Analysis (2020-2032)
7.5.2 Others Market Size Estimates and Forecasts to 2032 (USD Billion)
8. Wound Care Devices Market Segmentation, By Indication
8.1 Chapter Overview
8.2 Diabetic Ulcers
8.2.1 Diabetic Ulcers Market Trends Analysis (2020-2032)
8.2.2 Diabetic Ulcers Market Size Estimates and Forecasts to 2032 (USD Billion)
8.3 Surgical wounds
8.3.1 Surgical Wounds Market Trends Analysis (2020-2032)
8.3.2 Surgical Wounds Market Size Estimates and Forecasts to 2032 (USD Billion)
8.4 Pressure Ulcers
8.4.1 Pressure Ulcers Market Trends Analysis (2020-2032)
8.4.2 Pressure Ulcers Market Size Estimates and Forecasts to 2032 (USD Billion)
8.5 Others
8.5.1 Others Market Trends Analysis (2020-2032)
8.5.2 Others Market Size Estimates and Forecasts to 2032 (USD Billion)
9. Wound Care Devices Market Segmentation, By End-user
9.1 Chapter Overview
9.2 Hospitals
9.2.1 Hospitals Market Trends Analysis (2020-2032)
9.2.2 Hospitals Market Size Estimates and Forecasts to 2032 (USD Billion)
9.3 Homecare settings
9.3.1 Homecare settings Market Trends Analysis (2020-2032)
9.3.2 Homecare settings Market Size Estimates and Forecasts to 2032 (USD Billion)
9.4 Clinics
9.4.1 Clinics Market Trends Analysis (2020-2032)
9.4.2 Clinics Market Size Estimates and Forecasts to 2032 (USD Billion)
9.5 Others
9.5.1 Others Market Trends Analysis (2020-2032)
9.5.2 Others Market Size Estimates and Forecasts to 2032 (USD Billion)
10. Regional Analysis
10.1 Chapter Overview
10.2 North America
10.2.1 Trends Analysis
10.2.2 North America Wound Care Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.2.3 North America Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.2.4 North America Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.2.5 North America Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.2.6 USA
10.2.6.1 USA Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.2.6.2 USA Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.2.6.3 USA Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.2.7 Canada
10.2.7.1 Canada Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.2.7.2 Canada Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.2.7.3 Canada Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.2.8 Mexico
10.2.8.1 Mexico Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.2.8.2 Mexico Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.2.8.3 Mexico Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.3 Europe
10.3.1 Eastern Europe
10.3.1.1 Trends Analysis
10.3.1.2 Eastern Europe Wound Care Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.3.1.3 Eastern Europe Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.3.1.4 Eastern Europe Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.3.1.5 Eastern Europe Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.3.1.6 Poland
10.3.1.6.1 Poland Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.3.1.6.2 Poland Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.3.1.6.3 Poland Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.3.1.7 Romania
10.3.1.7.1 Romania Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.3.1.7.2 Romania Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.3.1.7.3 Romania Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.3.1.8 Hungary
10.3.1.8.1 Hungary Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.3.1.8.2 Hungary Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.3.1.8.3 Hungary Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.3.1.9 Turkey
10.3.1.9.1 Turkey Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.3.1.9.2 Turkey Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.3.1.9.3 Turkey Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.3.1.10 Rest of Eastern Europe
10.3.1.10.1 Rest of Eastern Europe Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.3.1.10.2 Rest of Eastern Europe Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.3.1.10.3 Rest of Eastern Europe Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.3.2 Western Europe
10.3.2.1 Trends Analysis
10.3.2.2 Western Europe Wound Care Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.3.2.3 Western Europe Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.3.2.4 Western Europe Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.3.2.5 Western Europe Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.3.2.6 Germany
10.3.2.6.1 Germany Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.3.2.6.2 Germany Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.3.2.6.3 Germany Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.3.2.7 France
10.3.2.7.1 France Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.3.2.7.2 France Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.3.2.7.3 France Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.3.2.8 UK
10.3.2.8.1 UK Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.3.2.8.2 UK Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.3.2.8.3 UK Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.3.2.9 Italy
10.3.2.9.1 Italy Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.3.2.9.2 Italy Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.3.2.9.3 Italy Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.3.2.10 Spain
10.3.2.10.1 Spain Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.3.2.10.2 Spain Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.3.2.10.3 Spain Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.3.2.11 Netherlands
10.3.2.11.1 Netherlands Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.3.2.11.2 Netherlands Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.3.2.11.3 Netherlands Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.3.2.12 Switzerland
10.3.2.12.1 Switzerland Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.3.2.12.2 Switzerland Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.3.2.12.3 Switzerland Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.3.2.13 Austria
10.3.2.13.1 Austria Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.3.2.13.2 Austria Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.3.2.13.3 Austria Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.3.2.14 Rest of Western Europe
10.3.2.14.1 Rest of Western Europe Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.3.2.14.2 Rest of Western Europe Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.3.2.14.3 Rest of Western Europe Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.4 Asia Pacific
10.4.1 Trends Analysis
10.4.2 Asia Pacific Wound Care Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.4.3 Asia Pacific Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.4.4 Asia Pacific Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.4.5 Asia Pacific Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.4.6 China
10.4.6.1 China Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.4.6.2 China Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.4.6.3 China Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.4.7 India
10.4.7.1 India Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.4.7.2 India Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.4.7.3 India Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.4.8 Japan
10.4.8.1 Japan Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.4.8.2 Japan Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.4.8.3 Japan Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.4.9 South Korea
10.4.9.1 South Korea Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.4.9.2 South Korea Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.4.9.3 South Korea Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.4.10 Vietnam
10.4.10.1 Vietnam Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.4.10.2 Vietnam Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.4.10.3 Vietnam Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.4.11 Singapore
10.4.11.1 Singapore Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.4.11.2 Singapore Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.4.11.3 Singapore Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.4.12 Australia
10.4.12.1 Australia Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.4.12.2 Australia Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.4.12.3 Australia Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.4.13 Rest of Asia Pacific
10.4.13.1 Rest of Asia Pacific Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.4.13.2 Rest of Asia Pacific Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.4.13.3 Rest of Asia Pacific Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.5 Middle East and Africa
10.5.1 Middle East
10.5.1.1 Trends Analysis
10.5.1.2 Middle East Wound Care Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.5.1.3 Middle East Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.5.1.4 Middle East Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.5.1.5 Middle East Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.5.1.6 UAE
10.5.1.6.1 UAE Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.5.1.6.2 UAE Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.5.1.6.3 UAE Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.5.1.7 Egypt
10.5.1.7.1 Egypt Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.5.1.7.2 Egypt Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.5.1.7.3 Egypt Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.5.1.8 Saudi Arabia
10.5.1.8.1 Saudi Arabia Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.5.1.8.2 Saudi Arabia Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.5.1.8.3 Saudi Arabia Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.5.1.9 Qatar
10.5.1.9.1 Qatar Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.5.1.9.2 Qatar Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.5.1.9.3 Qatar Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.5.1.10 Rest of Middle East
10.5.1.10.1 Rest of Middle East Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.5.1.10.2 Rest of Middle East Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.5.1.10.3 Rest of Middle East Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.5.2 Africa
10.5.2.1 Trends Analysis
10.5.2.2 Africa Wound Care Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.5.2.3 Africa Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.5.2.4 Africa Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.5.2.5 Africa Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.5.2.6 South Africa
10.5.2.6.1 South Africa Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.5.2.6.2 South Africa Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.5.2.6.3 South Africa Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.5.2.7 Nigeria
10.5.2.7.1 Nigeria Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.5.2.7.2 Nigeria Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.5.2.7.3 Nigeria Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.5.2.8 Rest of Africa
10.5.2.8.1 Rest of Africa Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.5.2.8.2 Rest of Africa Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.5.2.8.3 Rest of Africa Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.6 Latin America
10.6.1 Trends Analysis
10.6.2 Latin America Wound Care Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.6.3 Latin America Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.6.4 Latin America Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.6.5 Latin America Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.6.6 Brazil
10.6.6.1 Brazil Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.6.6.2 Brazil Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.6.6.3 Brazil Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.6.7 Argentina
10.6.7.1 Argentina Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.6.7.2 Argentina Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.6.7.3 Argentina Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.6.8 Colombia
10.6.8.1 Colombia Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.6.8.2 Colombia Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.6.8.3 Colombia Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.6.9 Rest of Latin America
10.6.9.1 Rest of Latin America Wound Care Devices Market Estimates and Forecasts, By Product Type (2020-2032) (USD Billion)
10.6.9.2 Rest of Latin America Wound Care Devices Market Estimates and Forecasts, By Indication (2020-2032) (USD Billion)
10.6.9.3 Rest of Latin America Wound Care Devices Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
11. Company Profiles
11.1 Smith & Nephew
11.1.1 Company Overview
11.1.2 Financial
11.1.3 Products/ Services Offered
11.1.4 SWOT Analysis
11.2 3M Healthcare
11.2.1 Company Overview
11.2.2 Financial
11.2.3 Products/ Services Offered
11.2.4 SWOT Analysis
11.3 ConvaTec Group plc
11.3.1 Company Overview
11.3.2 Financial
11.3.3 Products/ Services Offered
11.3.4 SWOT Analysis
11.4 Medtronic
11.4.1 Company Overview
11.4.2 Financial
11.4.3 Products/ Services Offered
11.4.4 SWOT Analysis
11.5 Molnlycke Health Care AB
11.5.1 Company Overview
11.5.2 Financial
11.5.3 Products/ Services Offered
11.5.4 SWOT Analysis
11.6 Coloplast A/S
11.6.1 Company Overview
11.6.2 Financial
11.6.3 Products/ Services Offered
11.6.4 SWOT Analysis
11.7 Hollister Incorporated
11.7.1 Company Overview
11.7.2 Financial
11.7.3 Products/ Services Offered
11.7.4 SWOT Analysis
11.8 Cardinal Health
11.8.1 Company Overview
11.8.2 Financial
11.8.3 Products/ Services Offered
11.8.4 SWOT Analysis
11.9 Derma Sciences, Inc.
11.9.1 Company Overview
11.9.2 Financial
11.9.3 Products/ Services Offered
11.9.4 SWOT Analysis
11.10 Kinetic Concepts, Inc.
11.10.1 Company Overview
11.10.2 Financial
11.10.3 Products/ Services Offered
11.10.4 SWOT Analysis
12. Use Cases and Best Practices
13. Conclusion
An accurate research report requires proper strategizing as well as implementation. There are multiple factors involved in the completion of good and accurate research report and selecting the best methodology to compete the research is the toughest part. Since the research reports we provide play a crucial role in any company’s decision-making process, therefore we at SNS Insider always believe that we should choose the best method which gives us results closer to reality. This allows us to reach at a stage wherein we can provide our clients best and accurate investment to output ratio.
Each report that we prepare takes a timeframe of 350-400 business hours for production. Starting from the selection of titles through a couple of in-depth brain storming session to the final QC process before uploading our titles on our website we dedicate around 350 working hours. The titles are selected based on their current market cap and the foreseen CAGR and growth.
The 5 steps process:
Step 1: Secondary Research:
Secondary Research or Desk Research is as the name suggests is a research process wherein, we collect data through the readily available information. In this process we use various paid and unpaid databases which our team has access to and gather data through the same. This includes examining of listed companies’ annual reports, Journals, SEC filling etc. Apart from this our team has access to various associations across the globe across different industries. Lastly, we have exchange relationships with various university as well as individual libraries.

Step 2: Primary Research
When we talk about primary research, it is a type of study in which the researchers collect relevant data samples directly, rather than relying on previously collected data. This type of research is focused on gaining content specific facts that can be sued to solve specific problems. Since the collected data is fresh and first hand therefore it makes the study more accurate and genuine.
We at SNS Insider have divided Primary Research into 2 parts.
Part 1 wherein we interview the KOLs of major players as well as the upcoming ones across various geographic regions. This allows us to have their view over the market scenario and acts as an important tool to come closer to the accurate market numbers. As many as 45 paid and unpaid primary interviews are taken from both the demand and supply side of the industry to make sure we land at an accurate judgement and analysis of the market.
This step involves the triangulation of data wherein our team analyses the interview transcripts, online survey responses and observation of on filed participants. The below mentioned chart should give a better understanding of the part 1 of the primary interview.

Part 2: In this part of primary research the data collected via secondary research and the part 1 of the primary research is validated with the interviews from individual consultants and subject matter experts.
Consultants are those set of people who have at least 12 years of experience and expertise within the industry whereas Subject Matter Experts are those with at least 15 years of experience behind their back within the same space. The data with the help of two main processes i.e., FGDs (Focused Group Discussions) and IDs (Individual Discussions). This gives us a 3rd party nonbiased primary view of the market scenario making it a more dependable one while collation of the data pointers.
Step 3: Data Bank Validation
Once all the information is collected via primary and secondary sources, we run that information for data validation. At our intelligence centre our research heads track a lot of information related to the market which includes the quarterly reports, the daily stock prices, and other relevant information. Our data bank server gets updated every fortnight and that is how the information which we collected using our primary and secondary information is revalidated in real time.

Step 4: QA/QC Process
After all the data collection and validation our team does a final level of quality check and quality assurance to get rid of any unwanted or undesired mistakes. This might include but not limited to getting rid of the any typos, duplication of numbers or missing of any important information. The people involved in this process include technical content writers, research heads and graphics people. Once this process is completed the title gets uploader on our platform for our clients to read it.
Step 5: Final QC/QA Process:
This is the last process and comes when the client has ordered the study. In this process a final QA/QC is done before the study is emailed to the client. Since we believe in giving our clients a good experience of our research studies, therefore, to make sure that we do not lack at our end in any way humanly possible we do a final round of quality check and then dispatch the study to the client.
By Product Type
Negative pressure Wound Therapy (NPWT)
Extracorporeal Shock Wave Therapy (ESWT)
Hyperbaric Oxygen Therapy (HBOT)
Others
By Indication
Diabetic Ulcers
Surgical wounds
Pressure Ulcers
Others
By End User
Hospitals
Homecare settings
Clinics
Others
Request for Segment Customization as per your Business Requirement: Segment Customization Request
REGIONAL COVERAGE:
North America
US
Canada
Mexico
Europe
Eastern Europe
Poland
Romania
Hungary
Turkey
Rest of Eastern Europe
Western Europe
Germany
France
UK
Italy
Spain
Netherlands
Switzerland
Austria
Rest of Western Europe
Asia Pacific
China
India
Japan
South Korea
Vietnam
Singapore
Australia
Rest of Asia Pacific
Middle East & Africa
Middle East
UAE
Egypt
Saudi Arabia
Qatar
Rest of the Middle East
Africa
Nigeria
South Africa
Rest of Africa
Latin America
Brazil
Argentina
Colombia
Rest of Latin America
Request for Country Level Research Report: Country Level Customization Request
Available Customization
With the given market data, SNS Insider offers customization as per the company’s specific needs. The following customization options are available for the report:
Product Analysis
Criss-Cross segment analysis (e.g. Product X Application)
Product Matrix which gives a detailed comparison of product portfolio of each company
Geographic Analysis
Additional countries in any of the regions
Company Information
Detailed analysis and profiling of additional market players (Up to five)
The Advanced Wound Care Market is valued USD 4.9 Billion in 2023 and anticipated to surpass USD 15.7 Billion, with growing CAGR of 4.8% by 2024-2031.
The Protein A Resin Market Size was valued at USD 1.34 billion in 2023, and is expected to reach USD 3.08 billion by 2032, and grow at a CAGR of 9.7% over the forecast period 2024-2032.
The Pharmacovigilance and Drug Safety Software Market Size was valued at USD 206.68 million in 2023 and is expected to reach USD 371.88 million by 2032 and grow at a CAGR of 6.76% over the forecast period 2024-2032.
The Hemoglobinopathies Market was valued at USD 9.63 billion in 2023 and is expected to reach USD 25.68 billion by 2032, growing at a CAGR of 11.56% from 2024 to 2032.
The Scoliosis Management Market size was USD 3060.51 Million in 2023 and is expected to Reach USD 4356.06 Million by 2032 and grow at a CAGR of 4% over the forecast period of 2024-2032.
The Protein Purification and Isolation Market size was estimated at USD 9.25 billion in 2023 and is expected to reach USD 23.10 billion by 2032 with a growing CAGR of 10.7% during the forecast period of 2024-2032.
Hi! Click one of our member below to chat on Phone