Get More Information on Toxoid Vaccine Market - Request Sample Report
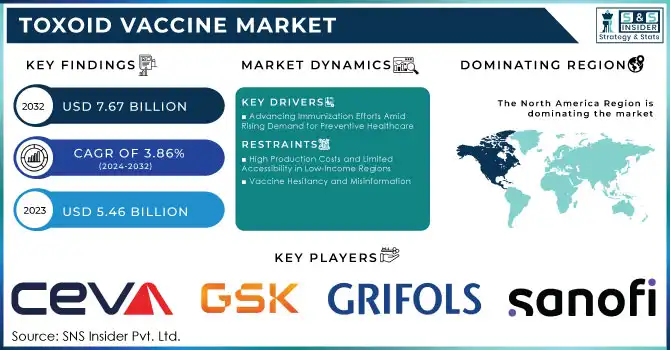
The Toxoid Vaccine Market size was worth US$ 5.46 billion in 2023 and is estimated to reach US$ 7.67 billion by 2032, growing at a CAGR of 3.86% during the forecast period 2024-2032.
The Toxoid Vaccine Market has been experiencing significant growth, driven by various global health initiatives, technological advancements, and increasing awareness about the importance of immunization in preventing infectious diseases. According to WHO, immunization prevents 2-3 million deaths every year from vaccine-preventable diseases, and the impact of toxoid vaccines in reducing these numbers is substantial.
The increasing prevalence of infectious diseases, along with the rise of public health initiatives, continues to drive demand for toxoid vaccines. Organizations like the World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) play a crucial role in supporting vaccination campaigns. For instance, the Global Vaccine Safety Initiative, launched by the WHO, emphasizes the need for continuous vaccination to reduce the global burden of vaccine-preventable diseases, especially in low- and middle-income countries.
Technological advancements in vaccine development have enhanced the safety, efficacy, and production capabilities of toxoid vaccines. The increase in government spending on healthcare and the shift toward preventive care are also key drivers. According to the CDC, tetanus vaccination rates have steadily remained above 90% in many developed countries, contributing to the decline in mortality from this disease. Routine childhood immunizations, which include toxoid vaccines, are becoming more widespread, contributing to the decline in vaccine-preventable diseases. Data from UNICEF shows that global immunization coverage reached 85% in 2022, with toxoid vaccines being a significant part of these programs. Additionally, studies published in PMC highlight that immunization programs have prevented over 30 million deaths in the last decade alone.
As healthcare systems continue to evolve and more innovative vaccination programs are introduced, the toxoid vaccine market is expected to continue its upward trajectory. The rising awareness of the long-term health benefits of immunization, alongside global health strategies focused on eradicating preventable diseases, will further propel market growth. Continued advancements in vaccine distribution and cold chain management will ensure broader access to toxoid vaccines, contributing to their increased adoption globally. Key trends such as the expansion of vaccine supply chains and the increasing focus on preventive healthcare are pivotal in shaping the future landscape of the toxoid vaccine market, making it a vital sector for improving public health outcomes worldwide.
Drivers
Advancing Immunization Efforts Amid Rising Demand for Preventive Healthcare
The Toxoid Vaccine Market is driven by several critical factors, including increasing investments in immunization infrastructure, advancements in vaccine research, and the growing emphasis on eradicating vaccine-preventable diseases globally. The rise of public-private partnerships, such as those facilitated by Gavi, the Vaccine Alliance, has enhanced vaccine accessibility in underserved regions, addressing immunization gaps in low-income countries. Additionally, governments across the globe are implementing mandatory vaccination policies and campaigns, particularly targeting newborns and school-age children, to reduce the incidence of diseases like tetanus and diphtheria.
Technological breakthroughs in biotechnology, such as recombinant DNA technology, have significantly improved the production and efficacy of toxoid vaccines. This has enabled the development of safer, more stable formulations with longer shelf lives, which are essential for distribution in remote areas. Furthermore, the growing focus on combination vaccines, which include toxoid components, reduces the number of injections needed, thereby improving compliance and coverage rates.
The increasing prevalence of antimicrobial resistance has heightened the importance of preventive measures like vaccination, positioning toxoid vaccines as a critical tool in combating bacterial infections. Rising awareness through initiatives such as World Immunization Week has also bolstered public understanding of vaccine benefits, driving higher adoption rates.
Moreover, the integration of advanced cold chain logistics has enhanced vaccine distribution efficiency, particularly in challenging environments. These factors collectively create a robust ecosystem for the toxoid vaccine market, ensuring its sustained growth and impact on global health.
Restraints
High Production Costs and Limited Accessibility in Low-Income Regions
The complex manufacturing processes of toxoid vaccines, including stringent quality control and cold chain requirements, contribute to high production costs. These factors, coupled with insufficient healthcare infrastructure in low-income countries, hinder vaccine distribution and accessibility.
Vaccine Hesitancy and Misinformation
Public resistance to vaccines, driven by misinformation and lack of awareness, continues to pose a significant challenge. Mistrust in healthcare systems and anti-vaccine movements further reduce immunization coverage, impacting the market's growth potential.
By Vaccine Type
In 2023, DTaP vaccines led the market with approximately 45.0% of the share, driven by their widespread use in routine childhood immunization programs. These vaccines are integral in protecting against three critical diseases—diphtheria, tetanus, and pertussis—making them a cornerstone of public health policies worldwide. Strong government support, high efficacy rates, and global efforts to curb infectious diseases contributed to their market dominance.
The TDaP vaccine segment is growing rapidly due to its increasing use in adolescent and adult booster immunizations, including recommendations for pregnant women to prevent pertussis in newborns. Expanded immunization guidelines and rising awareness about pertussis outbreaks have further driven the demand for TDaP vaccines.
By End Use
Hospitals accounted for approximately 55.0% of the market share in 2023, serving as the primary centers for vaccine administration. Their dominance is attributed to their advanced infrastructure, trained healthcare personnel, and critical role in managing both routine and emergency vaccination programs.
Government organizations are emerging as the fastest-growing segment, owing to their pivotal role in executing mass immunization campaigns. Partnerships with global health bodies and increased funding for national immunization programs have enabled these organizations to expand vaccine access, particularly in remote and underserved regions.
North America emerged as the dominant region in the toxoid vaccine market in 2023, driven by a combination of advanced healthcare systems and extensive immunization programs. The region benefits from high healthcare expenditure, which ensures the availability of vaccines to a broad population. Programs such as the Vaccines for Children (VFC) initiative in the U.S. have played a pivotal role in maintaining high immunization rates. Additionally, strong public awareness campaigns, robust government support, and established cold chain infrastructure for vaccine storage and distribution further solidify the region’s leadership. The presence of leading pharmaceutical players actively involved in vaccine development and distribution also gives North America a significant competitive edge.
Europe followed closely, with strong governmental policies mandating immunizations across various countries. Countries like Germany, France, and Italy have implemented stringent vaccination schedules, ensuring high coverage rates. Public health initiatives, including targeted campaigns to prevent diphtheria and tetanus outbreaks, further fuel market demand. Moreover, advanced healthcare infrastructure and collaborations with global organizations like the WHO bolster the region's market position.
Asia-Pacific stands out as the fastest-growing region over the forecast period, primarily due to large-scale immunization campaigns in populous countries like India and China. These nations have made significant strides in improving vaccine accessibility, particularly in rural and underserved areas. Governmental and non-governmental organizations such as UNICEF and the WHO play an instrumental role in these efforts, providing funding and logistical support. The growing prevalence of infectious diseases, coupled with improving healthcare infrastructure and increased public awareness about the importance of booster vaccines, is accelerating adoption. Urban and semi-urban areas in Asia-Pacific have also seen rising demand for toxoid vaccines due to enhanced healthcare access and awareness initiatives.
Need any customization research on Toxoid Vaccine Market - Enquiry Now
Pentavalent Vaccine (DTP-HepB-Hib), Tetanus Toxoid (TT) Vaccine
Ceva
Veterinary Toxoid Vaccines for Clostridial Diseases
GlaxoSmithKline plc (GSK)
Boostrix (TDaP Vaccine), Infanrix (DTaP Vaccine)
Grifols, S.A.
Tetanus Antitoxin
Zoetis Services LLC
Clostridial Toxoid Vaccines (e.g., Ultrabac series for livestock)
Adacel (TDaP Vaccine), DT Polio Tetanus (DT Vaccine), Pentaxim
Merck & Co., Inc.
Vaqta (Hepatitis A Vaccine with Toxoid components), Recombivax HB (combination vaccines with toxoid adjuvants)
Emergent BioSolutions Inc.
Tetanus and Diphtheria Toxoid Vaccines, BioThrax (Anthrax Vaccine Adsorbed)
Integrated BioTherapeutics, Inc.
Preclinical toxoid-based vaccine candidates for toxin-mediated diseases
Abbott
Paediatric DTP Vaccines
Avalon Pharma Private Limited
DTP and TT Vaccines for routine immunizations
Haffkine Bio-Pharmaceutical Corporation Ltd.
Tetanus Toxoid Vaccine, DTP Vaccine
Pfizer Inc.
Trumenba (meningococcal vaccine with toxoid components), Prevenar 13 (with toxoid as a carrier protein)
Virbac
Veterinary Toxoid Vaccines for Livestock
In Jan 2024, The National Pharmaceutical Pricing Authority (NPPA) granted an extension to the Serum Institute of India (SII) to continue manufacturing its two tetanus toxoid formulations until December 31, 2024. This decision comes after SII submitted a request to discontinue these formulations, seeking approval from the drug price regulator.
In Aug 2024, Pfizer's toxoid vaccine candidate (PF-06425090) for Clostridioides difficile failed to meet expectations in the Phase III CLOVER trial. The vaccine showed a 31% efficacy in reducing infection in adults aged 50 and older, but the result was not statistically significant.
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | US$ 5.46 Billion |
| Market Size by 2032 | US$ 7.67 Billion |
| CAGR | CAGR of 3.86% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Vaccine Type (Diphtheria, Tetanus, and Pertussis (DTaP), Diphtheria and Tetanus (DT), Tetanus, Diphtheria, and Pertussis (TDaP), Others) • By End Use (Hospitals, Specialty Centre, Governments Organization, Others) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | Bharat Biotech, Ceva, GlaxoSmithKline plc (GSK), Grifols S.A., Zoetis Services LLC, Sanofi, Merck & Co., Inc., Emergent BioSolutions Inc., Integrated BioTherapeutics Inc., Abbott, Avalon Pharma Private Limited, Haffkine Bio-Pharmaceutical Corporation Ltd., Pfizer Inc., and Virbac |
| Key Drivers | • Advancing Immunization Efforts Amid Rising Demand for Preventive Healthcare |
| Restraints | • High Production Costs and Limited Accessibility in Low-Income Regions • Vaccine Hesitancy and Misinformation |
Ans: The Toxoid Vaccine market is growing at a CAGR of 3.86% Over the Forecast Period 2024-2032.
The tetanus toxoid vaccine market is significantly impacted by COVID-19.
Ans: The global toxoid Vaccine Market size is expected to reach at USD 7.67 Bn by 2032.
The Increasing Burden of Tetanus Infectious are the fuel growth factors of Toxoid vaccine market.
Table of Contents
1. Introduction
1.1 Market Definition
1.2 Scope (Inclusion and Exclusions)
1.3 Research Assumptions
2. Executive Summary
2.1 Market Overview
2.2 Regional Synopsis
2.3 Competitive Summary
3. Research Methodology
3.1 Top-Down Approach
3.2 Bottom-up Approach
3.3. Data Validation
3.4 Primary Interviews
4. Market Dynamics Impact Analysis
4.1 Market Driving Factors Analysis
4.1.2 Drivers
4.1.2 Restraints
4.1.3 Opportunities
4.1.4 Challenges
4.2 PESTLE Analysis
4.3 Porter’s Five Forces Model
5. Statistical Insights and Trends Reporting
5.1 Incidence and Prevalence (2023)
5.2 Prescription Trends (2023), by Region
5.3 Device Volume, by Region (2020-2032)
5.4 Healthcare Spending, by Region (Government, Commercial, Private, Out-of-Pocket), 2023
6. Competitive Landscape
6.1 List of Major Companies, By Region
6.2 Market Share Analysis, By Region
6.3 Service Benchmarking
6.3.1 Service specifications and features
6.3.2 Pricing
6.4 Strategic Initiatives
6.4.1 Marketing and promotional activities
6.4.2 Distribution and Supply Chain Strategies
6.4.3 Expansion plans and new Service launches
6.4.4 Strategic partnerships and collaborations
6.5 Technological Advancements
6.6 Market Positioning and Branding
7. Toxoid Vaccine Market Segmentation, by Vaccine Type
7.2 Diphtheria, Tetanus, and Pertussis (DTaP)
7.2.1 Diphtheria, Tetanus, and Pertussis (DTaP) Market Trends Analysis (2020-2032)
7.2.2 Diphtheria, Tetanus, and Pertussis (DTaP) Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3 Diphtheria and Tetanus (DT)
7.3.1 Diphtheria and Tetanus (DT) Market Trends Analysis (2020-2032)
7.3.2 Diphtheria and Tetanus (DT) Market Size Estimates and Forecasts to 2032 (USD Billion)
7.4 Tetanus, Diphtheria, and Pertussis (TDaP)
7.4.1 Tetanus, Diphtheria, and Pertussis (TDaP) Market Trends Analysis (2020-2032)
7.4.2 Tetanus, Diphtheria, and Pertussis (TDaP) Market Size Estimates and Forecasts to 2032 (USD Billion)
7.5 Others
7.5.1 Others Market Trends Analysis (2020-2032)
7.5.2 Others Market Size Estimates and Forecasts to 2032 (USD Billion)
8. Toxoid Vaccine Market Segmentation, by End-use
8.2 Hospitals
8.2.1 Hospitals Market Trends Analysis (2020-2032)
8.2.2 Hospitals Market Size Estimates and Forecasts to 2032 (USD Billion)
8.3 Specialty Centre
8.3.1 Specialty Centre Market Trends Analysis (2020-2032)
8.3.2 Specialty Centre Market Size Estimates and Forecasts to 2032 (USD Billion)
8.4 Government Organization
8.4.1 Government Organization Market Trends Analysis (2020-2032)
8.4.2 Government Organization Market Size Estimates and Forecasts to 2032 (USD Billion)
8.5 Others
8.5.1 Others Market Trends Analysis (2020-2032)
8.5.2 Others Market Size Estimates and Forecasts to 2032 (USD Billion)
9. Regional Analysis
9.1 Chapter Overview
9.2 North America
9.2.1 Trends Analysis
9.2.2 North America Toxoid Vaccine Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.2.3 North America Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.2.4 North America Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.2.5 USA
9.2.5.1 USA Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.2.5.2 USA Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.2.6 Canada
9.2.6.1 Canada Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.2.6.2 Canada Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.2.7 Mexico
9.2.7.1 Mexico Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.2.7.2 Mexico Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.3 Europe
9.3.1 Eastern Europe
9.3.1.1 Trends Analysis
9.3.1.2 Eastern Europe Toxoid Vaccine Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.3.1.3 Eastern Europe Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.3.1.4 Eastern Europe Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.3.1.5 Poland
9.3.1.5.1 Poland Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.3.1.5.2 Poland Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.3.1.6 Romania
9.3.1.6.1 Romania Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.3.1.6.2 Romania Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.3.1.7 Hungary
9.3.1.7.1 Hungary Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.3.1.7.2 Hungary Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.3.1.8 Turkey
9.3.1.8.1 Turkey Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.3.1.8.2 Turkey Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.3.1.9 Rest of Eastern Europe
9.3.1.9.1 Rest of Eastern Europe Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.3.1.9.2 Rest of Eastern Europe Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.3.2 Western Europe
9.3.2.1 Trends Analysis
9.3.2.2 Western Europe Toxoid Vaccine Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.3.2.3 Western Europe Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.3.2.4 Western Europe Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.3.2.5 Germany
9.3.2.5.1 Germany Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.3.2.5.2 Germany Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.3.2.6 France
9.3.2.6.1 France Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.3.2.6.2 France Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.3.2.7 UK
9.3.2.7.1 UK Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.3.2.7.2 UK Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.3.2.8 Italy
9.3.2.8.1 Italy Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.3.2.8.2 Italy Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.3.2.9 Spain
9.3.2.9.1 Spain Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.3.2.9.2 Spain Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.3.2.10 Netherlands
9.3.2.10.1 Netherlands Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.3.2.10.2 Netherlands Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.3.2.11 Switzerland
9.3.2.11.1 Switzerland Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.3.2.11.2 Switzerland Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.3.2.12 Austria
9.3.2.12.1 Austria Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.3.2.12.2 Austria Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.3.2.13 Rest of Western Europe
9.3.2.13.1 Rest of Western Europe Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.3.2.13.2 Rest of Western Europe Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.4 Asia Pacific
9.4.1 Trends Analysis
9.4.2 Asia Pacific Toxoid Vaccine Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.4.3 Asia Pacific Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.4.4 Asia Pacific Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.4.5 China
9.4.5.1 China Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.4.5.2 China Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.4.6 India
9.4.5.1 India Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.4.5.2 India Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.4.5 Japan
9.4.5.1 Japan Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.4.5.2 Japan Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.4.6 South Korea
9.4.6.1 South Korea Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.4.6.2 South Korea Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.4.7 Vietnam
9.4.7.1 Vietnam Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.2.7.2 Vietnam Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.4.8 Singapore
9.4.8.1 Singapore Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.4.8.2 Singapore Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.4.9 Australia
9.4.9.1 Australia Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.4.9.2 Australia Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.4.10 Rest of Asia Pacific
9.4.10.1 Rest of Asia Pacific Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.4.10.2 Rest of Asia Pacific Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.5 Middle East and Africa
9.5.1 Middle East
9.5.1.1 Trends Analysis
9.5.1.2 Middle East Toxoid Vaccine Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.5.1.3 Middle East Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.5.1.4 Middle East Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.5.1.5 UAE
9.5.1.5.1 UAE Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.5.1.5.2 UAE Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.5.1.6 Egypt
9.5.1.6.1 Egypt Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.5.1.6.2 Egypt Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.5.1.7 Saudi Arabia
9.5.1.7.1 Saudi Arabia Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.5.1.7.2 Saudi Arabia Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.5.1.8 Qatar
9.5.1.8.1 Qatar Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.5.1.8.2 Qatar Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.5.1.9 Rest of Middle East
9.5.1.9.1 Rest of Middle East Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.5.1.9.2 Rest of Middle East Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.5.2 Africa
9.5.2.1 Trends Analysis
9.5.2.2 Africa Toxoid Vaccine Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.5.2.3 Africa Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.5.2.4 Africa Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.5.2.5 South Africa
9.5.2.5.1 South Africa Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.5.2.5.2 South Africa Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.5.2.6 Nigeria
9.5.2.6.1 Nigeria Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.5.2.6.2 Nigeria Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.6 Latin America
9.6.1 Trends Analysis
9.6.2 Latin America Toxoid Vaccine Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.6.3 Latin America Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.6.4 Latin America Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.6.5 Brazil
9.6.5.1 Brazil Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.6.5.2 Brazil Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.6.6 Argentina
9.6.6.1 Argentina Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.6.6.2 Argentina Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.6.7 Colombia
9.6.7.1 Colombia Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.6.7.2 Colombia Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.6.8 Rest of Latin America
9.6.8.1 Rest of Latin America Toxoid Vaccine Market Estimates and Forecasts, by Vaccine Type (2020-2032) (USD Billion)
9.6.8.2 Rest of Latin America Toxoid Vaccine Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
10. Company Profiles
10.1 Bharat Biotech
10.1.1 Company Overview
10.1.2 Financial
10.1.3 Products/ Services Offered
110.1.4 SWOT Analysis
10.2 GlaxoSmithKline plc (GSK)
10.2.1 Company Overview
10.2.2 Financial
10.2.3 Products/ Services Offered
10.2.4 SWOT Analysis
10.3 Ceva
10.3.1 Company Overview
10.3.2 Financial
10.3.3 Products/ Services Offered
10.3.4 SWOT Analysis
10.4 Grifols, S.A.
10.4.1 Company Overview
10.4.2 Financial
10.4.3 Products/ Services Offered
10.4.4 SWOT Analysis
10.5 Zoetis Services LLC
10.5.1 Company Overview
10.5.2 Financial
10.5.3 Products/ Services Offered
10.5.4 SWOT Analysis
10.6 Sanofi
10.6.1 Company Overview
10.6.2 Financial
10.6.3 Products/ Services Offered
10.6.4 SWOT Analysis
10.7 Merck & Co., Inc.
10.7.1 Company Overview
10.7.2 Financial
10.7.3 Products/ Services Offered
10.7.4 SWOT Analysis
10.8 Integrated BioTherapeutics, Inc.
10.8.1 Company Overview
10.8.2 Financial
10.8.3 Products/ Services Offered
10.8.4 SWOT Analysis
10.9 Emergent BioSolutions Inc.
10.9.1 Company Overview
10.9.2 Financial
10.9.3 Products/ Services Offered
10.9.4 SWOT Analysis
10.10 Pfizer Inc.
10.10.1 Company Overview
10.10.2 Financial
10.10.3 Products/ Services Offered
10.9.4 SWOT Analysis
11. Use Cases and Best Practices
12. Conclusion
An accurate research report requires proper strategizing as well as implementation. There are multiple factors involved in the completion of good and accurate research report and selecting the best methodology to compete the research is the toughest part. Since the research reports we provide play a crucial role in any company’s decision-making process, therefore we at SNS Insider always believe that we should choose the best method which gives us results closer to reality. This allows us to reach at a stage wherein we can provide our clients best and accurate investment to output ratio.
Each report that we prepare takes a timeframe of 350-400 business hours for production. Starting from the selection of titles through a couple of in-depth brain storming session to the final QC process before uploading our titles on our website we dedicate around 350 working hours. The titles are selected based on their current market cap and the foreseen CAGR and growth.
The 5 steps process:
Step 1: Secondary Research:
Secondary Research or Desk Research is as the name suggests is a research process wherein, we collect data through the readily available information. In this process we use various paid and unpaid databases which our team has access to and gather data through the same. This includes examining of listed companies’ annual reports, Journals, SEC filling etc. Apart from this our team has access to various associations across the globe across different industries. Lastly, we have exchange relationships with various university as well as individual libraries.

Step 2: Primary Research
When we talk about primary research, it is a type of study in which the researchers collect relevant data samples directly, rather than relying on previously collected data. This type of research is focused on gaining content specific facts that can be sued to solve specific problems. Since the collected data is fresh and first hand therefore it makes the study more accurate and genuine.
We at SNS Insider have divided Primary Research into 2 parts.
Part 1 wherein we interview the KOLs of major players as well as the upcoming ones across various geographic regions. This allows us to have their view over the market scenario and acts as an important tool to come closer to the accurate market numbers. As many as 45 paid and unpaid primary interviews are taken from both the demand and supply side of the industry to make sure we land at an accurate judgement and analysis of the market.
This step involves the triangulation of data wherein our team analyses the interview transcripts, online survey responses and observation of on filed participants. The below mentioned chart should give a better understanding of the part 1 of the primary interview.

Part 2: In this part of primary research the data collected via secondary research and the part 1 of the primary research is validated with the interviews from individual consultants and subject matter experts.
Consultants are those set of people who have at least 12 years of experience and expertise within the industry whereas Subject Matter Experts are those with at least 15 years of experience behind their back within the same space. The data with the help of two main processes i.e., FGDs (Focused Group Discussions) and IDs (Individual Discussions). This gives us a 3rd party nonbiased primary view of the market scenario making it a more dependable one while collation of the data pointers.
Step 3: Data Bank Validation
Once all the information is collected via primary and secondary sources, we run that information for data validation. At our intelligence centre our research heads track a lot of information related to the market which includes the quarterly reports, the daily stock prices, and other relevant information. Our data bank server gets updated every fortnight and that is how the information which we collected using our primary and secondary information is revalidated in real time.

Step 4: QA/QC Process
After all the data collection and validation our team does a final level of quality check and quality assurance to get rid of any unwanted or undesired mistakes. This might include but not limited to getting rid of the any typos, duplication of numbers or missing of any important information. The people involved in this process include technical content writers, research heads and graphics people. Once this process is completed the title gets uploader on our platform for our clients to read it.
Step 5: Final QC/QA Process:
This is the last process and comes when the client has ordered the study. In this process a final QA/QC is done before the study is emailed to the client. Since we believe in giving our clients a good experience of our research studies, therefore, to make sure that we do not lack at our end in any way humanly possible we do a final round of quality check and then dispatch the study to the client.
Diphtheria, Tetanus, and Pertussis (DTaP)
Diphtheria and Tetanus (DT)
Tetanus, Diphtheria, and Pertussis (TDaP)
Others
By End Use
Hospitals
Specialty Centre
Government Organization
Others
Request for Segment Customization as per your Business Requirement: Segment Customization Request
Regional Coverage:
North America
US
Canada
Mexico
Europe
Eastern Europe
Poland
Romania
Hungary
Turkey
Rest of Eastern Europe
Western Europe
Germany
France
UK
Italy
Spain
Netherlands
Switzerland
Austria
Rest of Western Europe
Asia Pacific
China
India
Japan
South Korea
Vietnam
Singapore
Australia
Rest of Asia Pacific
Middle East & Africa
Middle East
UAE
Egypt
Saudi Arabia
Qatar
Rest of the Middle East
Africa
Nigeria
South Africa
Rest of Africa
Latin America
Brazil
Argentina
Colombia
Rest of Latin America
Request for Country Level Research Report: Country Level Customization Request
Available Customization
With the given market data, SNS Insider offers customization as per the company’s specific needs. The following customization options are available for the report:
Product Analysis
Criss-Cross segment analysis (e.g. Product X Application)
Product Matrix which gives a detailed comparison of the product portfolio of each company
Geographic Analysis
Additional countries in any of the regions
Company Information
Detailed analysis and profiling of additional market players (Up to five)
The Ambulance Equipment Market was valued at USD 5.13 billion in 2023 and is expected to reach USD 7.23 billion by 2032, growing at a CAGR of 3.89% over the forecast period of 2024-2032.
The Breast Cancer Liquid Biopsy Market was valued at USD 1.13 billion in 2023 and is expected to reach USD 2.37 billion by 2032, growing at a CAGR of 8.61% over the forecast period of 2024-2032.
The Chromatography Instruments Market size was valued at USD 9.47 billion in 2023, expected to reach USD 14.90 billion by 2032 at CAGR 5.19% from 2024-2032.
The Genomic Medicine Market was valued at USD 25.35 billion in 2023 and is projected to reach USD 86.35 billion by 2032, expanding at a CAGR of 14.64%.
The Cancer biomarkers market Size was valued at USD 22 billion in 2023, and is expected to reach USD 58.12 billion by 2032, and grow at a CAGR of 11.4% over the forecast period 2024-2032.
The Analgesics Market was estimated at USD 47.32 billion in 2023 and is expected to reach at USD 75.73 billion by 2032, and develop at a CAGR of 5.39% over the forecast period 2024-2032.
Hi! Click one of our member below to chat on Phone