Stroke Diagnostics and Therapeutics Market Report Scope & Overview:
Get More Information on Stroke Diagnostics and Therapeutics Market - Request Sample Report
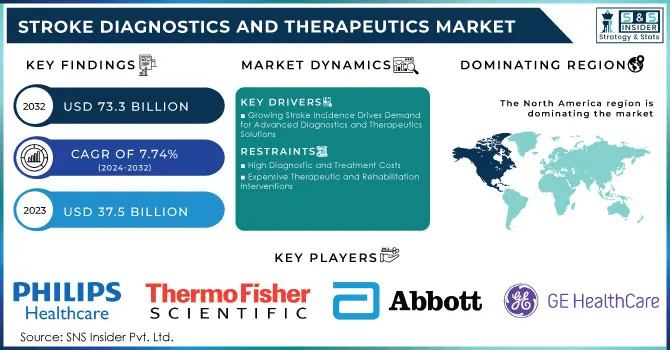
The Stroke Diagnostics and Therapeutics Market was valued at USD 37.5 Billion in 2023 and is expected to reach USD 73.3 Billion by 2032 and grow at a CAGR of 7.74% over the forecast period of 2024-2032.
Stroke diagnostics and therapeutics are the most promising markets with significant growth, led mainly by increased cases of ischemic strokes. According to the CDC, 795,000 people suffer from a new or recurrent stroke every year in the United States, which was due to an ischemic stroke in 87% of the cases. Increasing cases of stroke, especially ischemic stroke due to arterial blockage, make for a compelling case for more advanced diagnostics as well as novel treatments.
Technological developments in imaging, among them CT and Magnetic Resonance Imaging (MRI) scans, have significantly improved the accuracy and speed of stroke diagnosis; and demand throughout much of the world for such technologies grows daily. Imaging innovations such as perfusion and diffusion-weighted imaging allow neurologists to ascertain damage to brain tissue more precisely, which might now result in more favorable outcomes because of improved treatment. Integration of telemedicine into stroke care has been introduced as a means of expanding access to expert consultations, especially where needed, in underserved regions, and improving patient outcomes.
Public health initiatives have educated the masses on the symptoms of a stroke, which enables patients to access a health facility in the early stages, thus increasing diagnosis rates. The research into stroke management is ongoing, and new therapeutic interventions such as clot-dissolving drugs and personalized rehabilitation have been established, hence increasing market growth.
Hence, despite this upward trend, high-end diagnostic equipment and therapy-priced treatments remain a major challenge. Advanced MRI and CT scanners, operational costs, and maintenance costs act as major impediments to the growth of the market. Huge monetary limitations amongst patients themselves also exist, especially in those cases where patients are not sufficiently covered by insurance programs, limiting them from availing of stroke care in an outright and comprehensive manner.
However, the market will keep moving due to the emphasis placed on early detection, personalized medicine, and telehealth solutions. Rising incidences of strokes, combined with other developments in diagnostics and therapeutics, are expected to cause tremendous growth in the stroke diagnostics and treatment market during the next few years.
|
Diagnostic Method (for Stroke) |
Description |
Advantages |
Limitations |
|---|---|---|---|
|
CT Scan |
Imaging to detect brain abnormalities |
Fast and widely available |
Limited in detecting small strokes |
|
MRI |
High-resolution imaging for detailed brain structure |
Superior detail and sensitivity |
Longer time required for scanning |
|
Ultrasound |
Used to examine blood flow in the carotid arteries |
Non-invasive and cost-effective |
Limited to superficial blood flow |
|
PET Scan |
Functional imaging that detects metabolic activity |
Useful for identifying tissue viability |
High cost and less availability |
|
Angiography |
Imaging blood vessels using contrast dye |
Excellent for visualizing blockages |
Invasive and carries some risk |
|
Therapeutic Approach (in stroke) |
Description |
Indications |
Common Medications |
|---|---|---|---|
|
Thrombolysis |
Use of clot-dissolving drugs to restore blood flow |
Ischemic stroke |
tPA (Tissue Plasminogen Activator) |
|
Antiplatelet Therapy |
Prevention of further clots by inhibiting platelet aggregation |
Post-stroke prevention |
Aspirin, Clopidogrel |
|
Anticoagulants |
Blood thinners to prevent clot formation |
Atrial fibrillation and DVT |
Warfarin, Rivaroxaban |
|
Surgical Intervention |
Procedures to remove clots or repair blood vessels |
Severe cases and hemorrhagic strokes |
Endovascular procedures |
|
Rehabilitation Therapy |
Physical and occupational therapy for recovery |
Post-stroke recovery |
Customized rehabilitation programs |
Stroke Diagnostics and Therapeutics Market Dynamics
Drivers
-
Growing Stroke Incidence Drives Demand for Advanced Diagnostics and Therapeutics Solutions
Increasing stroke cases across the global population provide an initial impetus for market growth in the Stroke Diagnostics and Therapeutics Market. Contributing factors such as the aging demographic, sedentary lifestyles, and elevated rates of high blood pressure, diabetes, and obesity are fueling increased demand for healthcare services related to stroke. According to the World Health Organization's Global Stroke Factsheet, lifetime stroke risk has risen by 50% in the past 17 years. Presently, it has been observed that 1 in every 4 people will suffer from a stroke at some point during their lifetime.
This would further enhance the incidence of stroke and all of its complications in the aged population of the developed world, increasing the demand for a sophisticated diagnostic tool, new treatment options, and comprehensive rehabilitation solutions. Apart from this, increased awareness programs relating to the prevention of stroke, risk factors, and early warning signs for stroke are enhancing early diagnosis and timely medical intervention, hence further augmenting the demand for effective stroke care solutions worldwide.
A leading trend in the stroke diagnostics and therapeutics market is the increasing use of artificial intelligence and machine learning algorithms for stroke diagnosis and imaging analysis. AI-based platforms can rapidly analyze medical images such as CT scans and MRIs to detect slight stroke indicators find areas that suffer from ischemia and enable differentiation between various stroke subtypes. This technology aids radiologists and neurologists in the interpretation of complex imaging data and thus substantially reduces the time taken to interpret, improving diagnostic accuracy.
The expansion of telemedicine and remote stroke care is altering the market landscape. Digital biomarkers and remote monitoring technologies are gaining acceptability. They allow continuous follow-up with a stroke patient's health. These will facilitate early detection of complications, risk assessment for recurrence, and implementation of individualized treatment strategies based on real-time analytics. Remote stroke management is thus more efficient and accessible.
Restraints
-
High Diagnostic and Treatment Costs
-
Expensive Therapeutic and Rehabilitation Interventions
-
Stroke Diagnostics and Therapeutics Market Segmentation Overview
By Type
The stroke diagnostics and therapeutics market is subdivided into two main categories: diagnostics and therapeutics. From these two categories, in 2023 therapeutics has accounted for the highest share of revenue at 57.3% of the overall market revenue. This can be mainly ascribed to immediate therapy post stroke where therapeutic interventions are given greater acuteness to stabilize the patient's condition. The therapeutics segment is further promoted by the easy availability of diverse treatment options, including medications, surgical procedures, and rehabilitation therapies.
On the other hand, diagnostics will witness the highest growth during the forecast period. Increasing appreciation of the importance of early stroke detection and timely, accurate diagnosis, there is an increasing desire for more advanced diagnostic tools and technologies, including imaging modalities, biomarker analysis, and genetic testing. All of these advance the diagnostic timeline, which reduces long-term complications and improves patient outcomes. The growth of this segment is further catalyzed by the increasing adoption of non-invasive and efficient diagnostic techniques.
By Application
Based on Application, the stroke diagnostics and therapeutics market can be divided into two types: ischemic stroke and hemorrhagic stroke. Amongst them, the ischemic stroke segment held the largest market share of 76.4% in 2023. This is mainly because the number of patients suffering from ischemic stroke is much more as compared to hemorrhagic strokes. The research findings by Neurology Clinics indicate that ischemic strokes are responsible for 70% to 80% of total deaths as a result of stroke in the United States. Since it is anticipated that the incidence of stroke, on average, will continue to increase, the segment for ischemic stroke is likely to increase at a stable rate over the next few years.
However, the hemorrhagic stroke segment is likely to be the largest gainer during the forecasting period. Even though the incidence is relatively low, there is a higher risk of death and permanent disability associated with hemorrhagic stroke. Growth in this area is primarily driven by further developments in diagnostic imaging and therapeutic interventions as well as growing interest in more timely diagnosis and treatment of hemorrhagic stroke. More importantly, rising awareness about the severe health impact of hemorrhagic strokes is fueling healthcare spending as well as the need for advanced stroke care solutions, which in turn is driving fast growth in the segment.
Stroke Diagnostics and Therapeutics Market Regional Analysis
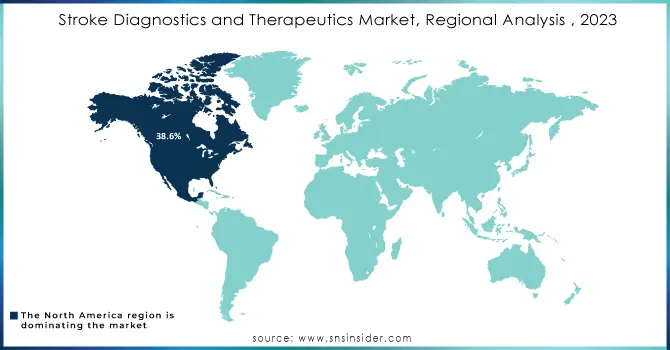
The North American region continued to be a leader in the global market, accounting for 38.6% of the shares in the year 2023. For example, in the United States, a stroke occurs every 40 seconds, and somebody dies due to a stroke every 3 minutes and 14 seconds, as reported by the Centers for Disease Control and Prevention. Growth in the North American region is contributed to by a wide variety of factors like a rising incidence of strokes in the U.S. and Canada, thereby leading to an increased demand for diagnostic and therapeutic solutions. The growth rate in North America is driven strongly by the rapidly increasing elderly population; stroke incidence is far higher among older adults than in any other age group, driving the North American market forward.
The Asia Pacific region is expected to show the highest compound annual growth rate (CAGR) during the forecast period. Factors driving this growth include increased patient awareness and an upsurge in the level of healthcare expenditure in the major countries, China, Japan, and India. Stroke prevention is one of the main focus areas with early diagnosis and a developing infrastructure of healthcare facilities providing an inviting environment for the growth of the Stroke Diagnostics and Therapeutics Market in the Asia Pacific region.
Need Any Customization Research On Stroke Diagnostics Market - Inquiry Now
Key Players in Stroke Diagnostics Market
-
Philips Healthcare
-
Roche Diagnostics
-
Abbott Laboratories
-
Siemens Healthineers
-
GE Healthcare
-
Bayer
-
BrainHQ
-
Cordis Corporation
Key Players in Stroke Therapeutics Market
-
Stryker Corporation
-
Pfizer
-
Genentech, Inc.
-
Medtronic plc
-
Merck & Co. Inc
-
Siemens AG
-
Koninklijke Philips N.V.
Recent Developments
In Oct 2024, Siemens Healthineers partnered with over 20 industry and public organizations, including seven top stroke hospitals, to co-lead the UMBRELLA1 project aimed at improving stroke care across Europe. The €26.9 million initiative is funded by the Innovative Health Initiative (IHI) and industry partners, focusing on enhancing stroke management and patient outcomes over the next five years.
In May 2024, Midland Pharmaceuticals emerged as a new player in the market, focusing on the development of innovative therapeutics for spinal cord injuries and strokes. This company aims to advance treatment options in these critical areas, contributing to the ongoing evolution of stroke diagnostics and therapeutics.
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | US$ 37.5 billion |
| Market Size by 2032 | US$ 73.3 billion |
| CAGR | CAGR of 7.74% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Type [Diagnostics (Imaging Diagnostics, Blood Tests, Others), Therapeutics (Acute Stroke Therapies, Secondary Prevention Therapies, Neurorehabilitation)] • By Application (Haemorrhagic Stroke, Ischemic Stroke) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | Philips Healthcare, Roche Diagnostics, Thermo Fisher Scientific, Abbott Laboratories, Siemens Healthineers, GE Healthcare, Bayer, BrainHQ, Cordis Corporation, Stryker Corporation, Pfizer, Boston Scientific Corporation, Genentech, Inc., Medtronic plc, Merck & Co. Inc, Siemens AG, Koninklijke Philips N.V. |
| Key Drivers | • Growing Stroke Incidence Drives Demand for Advanced Diagnostics and Therapeutics Solutions |
| Restraints | • High Diagnostic and Treatment Costs • Expensive Therapeutic and Rehabilitation Interventions |

