To Get More Information on Sleep Apnea Devices Market - Request Sample Report
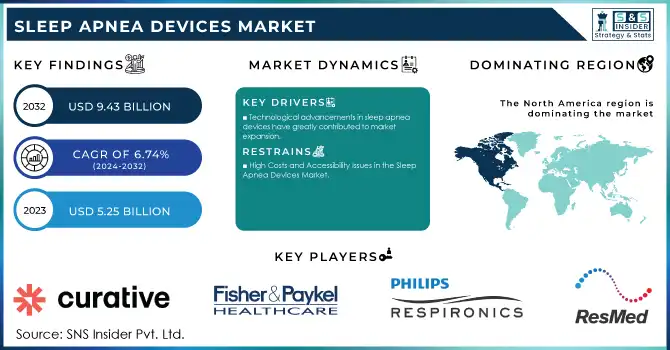
The Sleep Apnea Devices Market size was valued at USD 5.25 billion in 2023 and is projected to reach USD 9.43 billion by 2032, growing at a CAGR of 6.74% over the forecast period 2024-2032.
The Sleep Apnea Devices Market is experiencing significant growth, largely driven by the rising prevalence of sleep apnea, growing public awareness, and advancements in treatment technologies. Sleep apnea affects millions worldwide, particularly obstructive sleep apnea. According to the National Sleep Foundation, approximately 22 million Americans suffer from sleep apnea, with a staggering 80% of moderate and severe cases remaining undiagnosed. Globally, the World Health Organization estimates that 936 million people aged 30-69 are affected by sleep apnea, with key contributing factors such as obesity—affecting over 40% of U.S. adults—and an aging population. These statistics underscore the growing need for effective treatment options.
Technological advancements have played a pivotal role in the market’s expansion. Continuous Positive Airway Pressure machines, Bi-level Positive Airway Pressure devices, and Automatic Positive Airway Pressure machines have evolved to be more compact, portable, and comfortable, addressing patient compliance challenges. For example, the Somnetics Transcend 3 mini CPAP offers a portable solution for patients needing CPAP therapy on the go. Innovations like these make it easier for individuals to manage their condition at home and while traveling. Additionally, the FDA’s recent approval of the Oral Appliance for Severe Sleep Apnea Therapy represents a significant shift in treatment options, providing an alternative for patients who struggle with traditional CPAP devices.
Awareness campaigns by organizations like the American Academy of Sleep Medicine and the Centers for Disease Control and Prevention have significantly contributed to increasing public knowledge about sleep apnea and its associated risks. Through initiatives like the Obstructive Sleep Apnea Awareness Project, these organizations have helped educate the public on the importance of early diagnosis and treatment. This heightened awareness has led to a growing number of people seeking sleep apnea screenings and treatments.
The integration of telemedicine and mobile health technologies has further transformed the market. Platforms like SleepCycle and ApneaLink offer remote monitoring and diagnostic services, allowing patients to track their sleep patterns and receive timely feedback on their condition. These innovations are especially beneficial for individuals in remote areas or those with limited access to traditional healthcare facilities.
Drivers
Awareness of Health Risks and Public Health Campaigns
The growing awareness of the health risks associated with untreated sleep apnea is a significant driver of market growth. As sleep apnea is linked to serious conditions such as cardiovascular disease, stroke, and hypertension, more individuals are seeking early diagnosis and treatment. Medical professionals and advocacy groups, including the American Academy of Sleep Medicine, have played a pivotal role in educating the public on these risks. Public health campaigns have been instrumental in encouraging individuals to take proactive steps in managing their sleep apnea, leading to increased acceptance of sleep apnea therapies. This heightened awareness is driving demand for early intervention and treatment options, fueling the market for sleep apnea devices.
Technological advancements in sleep apnea devices have greatly contributed to market expansion.
Manufacturers have focused on developing more effective, user-friendly, and comfortable treatment options. Innovations such as quieter and more compact CPAP machines have made treatment easier and more accessible, especially for individuals who previously found the devices cumbersome. Portable CPAP machines designed for travel have made it easier for patients to comply with therapy even when away from home. Additionally, the rise of non-invasive treatments, such as oral appliances, offers alternative solutions for patients who may find traditional CPAP machines uncomfortable. These technological improvements are enhancing patient compliance and contributing to the growth of the sleep apnea device market.
The improvement in reimbursement policies and insurance coverage is another key factor driving the growth of the sleep apnea device market.
In the United States, Medicare and private insurance plans now cover home sleep apnea testing and the purchase of treatment devices, making these products more affordable and accessible to a wider range of patients. Furthermore, integrating mobile health solutions and telemedicine has allowed patients to monitor and manage their condition remotely, enhancing the overall treatment experience. Remote monitoring through mobile applications has made it easier for healthcare providers to track progress, adjust treatment plans, and ensure better patient outcomes. This combination of improved reimbursement and technological integration is accelerating market growth.
Restraints
High Costs and Accessibility Issues in the Sleep Apnea Devices Market
One of the primary restraints in the Sleep Apnea Devices Market is the high cost of advanced treatment devices such as CPAP and BiPAP machines. While these devices are essential for managing sleep apnea, the initial purchase cost and maintenance expenses can be prohibitive for many individuals, particularly in lower-income regions or developing countries. Despite improvements in insurance coverage, many patients still face substantial out-of-pocket expenses. This financial burden, combined with limited insurance reimbursement in certain areas, restricts access to necessary devices for a large segment of the population. As a result, the affordability factor becomes a key barrier, preventing widespread adoption of sleep apnea treatments, especially for those with insufficient financial resources.
Patient Compliance Challenges in Sleep Apnea Treatment
Another significant challenge facing the Sleep Apnea Devices Market is patient non-compliance with traditional treatments, particularly CPAP therapy. While CPAP machines are the gold standard for sleep apnea treatment, they often come with discomfort, including noise, air pressure issues, and difficulty with mask fit. Many users find it hard to adapt to these machines, leading to poor adherence rates. This non-compliance is one of the major reasons why the effectiveness of sleep apnea treatments is sometimes limited. The discomfort associated with continuous use can result in patients discontinuing their treatment or using the devices intermittently, reducing the overall success rate. Additionally, the complexity of some devices may deter patients from fully committing to therapy, impacting market growth.
By Product
In 2023, therapeutic devices held the largest share of the Sleep Apnea Devices Market, accounting for approximately 65% of the market revenue. The dominance of this segment is attributed to the rising adoption of CPAP machines, BiPAP machines, and oral appliances, which are widely recognized as effective treatment options for managing sleep apnea. Technological advancements, such as quieter and more portable devices, have further increased patient preference for therapeutic devices. Additionally, the growing awareness about the adverse health impacts of untreated sleep apnea has led to increased usage of therapeutic solutions for timely intervention.
The diagnostic devices segment is expected to witness the fastest growth during the forecast period, driven by an increasing emphasis on early detection of sleep apnea. Portable home sleep testing devices are gaining traction due to their convenience, cost-effectiveness, and ability to deliver accurate results. The adoption of diagnostic devices is further supported by insurance coverage policies and public health initiatives promoting early diagnosis.
By Age Group
The age group of 40–60 years dominated the market in 2023, contributing approximately 55% of the overall revenue. This age group is at a higher risk of developing sleep apnea due to lifestyle factors, comorbidities like obesity and hypertension, and natural aging processes. Increased health awareness among this demographic and better access to diagnostic and therapeutic solutions have strengthened its market dominance.
The above-60 age group is anticipated to grow at the highest rate, fueled by the increasing prevalence of sleep apnea among older adults. Aging-related factors, such as reduced muscle tone in the airway and a higher likelihood of coexisting conditions like cardiovascular diseases, contribute to the demand for sleep apnea devices in this segment. Moreover, improvements in healthcare infrastructure and insurance coverage are making these devices more accessible to elderly populations.
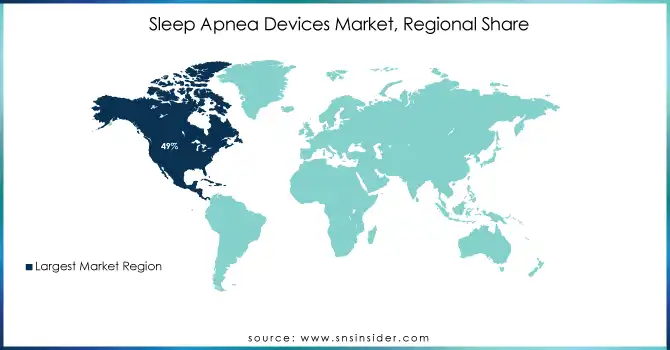
In 2023, North America emerged as the leading region in the Sleep Apnea Devices Market, holding the largest share of 49%. This dominance is attributed to the high prevalence of sleep apnea, advanced healthcare infrastructure, and widespread adoption of innovative devices. The region benefits from an extensive network of sleep centers, rising public awareness initiatives, and supportive reimbursement policies. The United States spearheads this growth, driven by robust R&D investments, frequent regulatory approvals, and early adoption of cutting-edge technologies.
Europe secured the second-largest market share, supported by increasing awareness of the health risks associated with untreated sleep apnea and proactive government measures encouraging early diagnosis and treatment. Key contributors, such as Germany and the United Kingdom, leverage their well-established healthcare systems and growing emphasis on non-invasive treatment options to drive market expansion.
The Asia-Pacific region is poised for the fastest growth during the forecast period, fueled by a rising population, an increasing prevalence of sleep apnea, and improved access to healthcare services. Nations like China, India, and Japan are at the forefront of this regional surge, backed by rapid urbanization, growing healthcare spending, and portable and affordable diagnostic device availability. These factors make Asia-Pacific a critical area for market development, offering significant opportunities for industry stakeholders.
Do You Need any Customization Research on Sleep Apnea Devices Market - Enquire Now
Continuous Positive Airway Pressure (CPAP) devices
Automatic Positive Airway Pressure (APAP) devices
Bilevel Positive Airway Pressure (BiPAP) devices
Masks and accessories
Cloud-connected software solutions
Respironics (a subsidiary of Koninklijke Philips N.V.)
DreamStation CPAP and BiPAP machines
DreamWear nasal and full-face masks
Sleep therapy tracking tools
Fisher & Paykel Healthcare Limited
SleepStyle CPAP machines
Evora nasal masks
Vitera full-face masks
Humidifiers and breathing circuits
Curative Medical, Inc.
CPAP and BiPAP machines
Portable sleep therapy devices
Sleep diagnostic systems
React Health (Respiratory Product Line from Invacare Corporation)
Sleep therapy solutions (CPAP, BiPAP devices)
Masks and accessories
Somnetics International, Inc.
Transcend portable CPAP devices
Sleep apnea therapy accessories
BMC Medical Co., Ltd.
G3 CPAP series
Masks and sleep diagnostic devices
Non-invasive ventilators
Natus Medical Incorporated
Sleep diagnostic systems
EEG and polysomnography equipment
SOMNOmedics GmbH
Portable sleep diagnostic devices
Home sleep testing solutions
Compumedics Limited
Somte PSG diagnostic systems
Grael polysomnography systems
Itamar Medical Ltd.
WatchPAT sleep apnea diagnostic devices
Home sleep testing solutions
Nihon Kohden Corporation
Polysomnography systems
Sleep lab diagnostic tools
OpenAirway
Oral appliances for sleep apnea
Non-invasive airway management solutions
Cadwell Industries, Inc.
Polysomnography systems
Diagnostic tools for sleep studies
SomnoMed
Oral appliance therapy devices
Customized mandibular advancement splints
Braebon Medical Corporation
Sleep diagnostic and therapy systems
PSG accessories and sensors
In Dec 2024, Eli Lilly's weight-loss drug, Zepbound, received approval from the U.S. Food and Drug Administration (FDA) as the first-ever medication to treat obstructive sleep apnea directly. This groundbreaking approval marks a significant milestone in addressing the common sleeping disorder through pharmaceutical intervention.
In Oct 2024, LuxCreo, Inc., a leader in personalized medical and dental device manufacturing, collaborated with EMA Sleep, Inc. to enable same-day, in-clinic, and scalable production of advanced EMA devices for treating Obstructive Sleep Apnea (OSA). This partnership leverages cutting-edge 3D printing technology to enhance patient care and streamline manufacturing processes.
In April 2024, Philips disclosed that the costs associated with a consent decree issued by a U.S. court, which imposed restrictions on producing its sleep apnea devices, resulted in a provision of USD 378 million for Q4 2023. The company projected these costs to account for approximately 1% of its total revenue in 2024.
| Report Attributes | Details |
| Market Size in 2023 | USD 5.25 billion |
| Market Size by 2032 | USD 9.43 billion |
| CAGR | CAGR of 6.74% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Product [Therapeutic Devices (PAP Devices {CPAP Devices, APAP Devices, BPAP Devices}, Facial Interfaces) Masks {Full-face Masks, Nasal Pillow Masks, Nasal Masks}, Cushions), Accessories (Humidifiers Accessories, Power Accessories, Transportation Accessories, Communication Accessories, Chin Restraints, Other Accessories), Oral Appliances (Mandibular Advancement Devices, Tongue-retaining Devices, Daytime-Nighttime Appliances), Other Therapeutic Devices, [Diagnostic Devices, (PSG Devices {Ambulatory PSG Devices, Clinical PSG Devices}, Home Sleep Testing Devices, Oximeters (Fingertip Oximeters, Handheld Oximeters, Wrist-worn Oximeters, Tabletop Oximeters, {Actigraphy Systems, Sleep Screening Devices})] •By Age Group [Below 40 Years, 40-60 Years, Above 60 Years] •By Gender [Male Patients, Female Patients] •By End User [Sleep Laboratories, Clinics, and Hospitals, Home Care Settings/ Individuals] |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | ResMed, Respironics (Philips), Fisher & Paykel Healthcare, Curative Medical, React Health, Somnetics, BMC Medical, Natus Medical, SOMNOmedics, Compumedics, Itamar Medical, Nihon Kohden, OpenAirway, Cadwell Industries, SomnoMed, Braebon Medical. |
| Key Drivers | • Awareness of Health Risks and Public Health Campaigns • Technological Innovations and Enhanced Treatment Options • Improved Reimbursement Policies and Integration of Mobile Health |
| Restraints | • High Costs and Accessibility Issues in the Sleep Apnea Devices Market • Patient Compliance Challenges in Sleep Apnea Treatment |
Ans: The size of Sleep Apnea Devices market is approx. USD 5.25 billion in 2023.
Ans: The CAGR Growth rate of Sleep Apnea Devices Market is approx. at a CAGR of 6.74% from 2024 to 2032.
Ans: Some key players operating in the sleep apnea devices market include Curative Medical Inc.; Philips Respironics; ResMed; Fisher & Paykel Healthcare; Cadwell Laboratories; and Invacare Corporation.
Ans: The rising prevalence of obstructive sleep apnea (OSA) patients is one of the major reasons fuelling the growth of the sleep apnea devices market.
Table of Content
1. Introduction
1.1 Market Definition
1.2 Scope (Inclusion and Exclusions)
1.3 Research Assumptions
2. Executive Summary
2.1 Market Overview
2.2 Regional Synopsis
2.3 Competitive Summary
3. Research Methodology
3.1 Top-Down Approach
3.2 Bottom-up Approach
3.3. Data Validation
3.4 Primary Interviews
4. Market Dynamics Impact Analysis
4.1 Market Driving Factors Analysis
4.1.1 Drivers
4.1.2 Restraints
4.1.3 Opportunities
4.1.4 Challenges
4.2 PESTLE Analysis
4.3 Porter’s Five Forces Model
5. Statistical Insights and Trends Reporting
5.1 Incidence and Prevalence (2023)
5.2 Prescription Trends (2023), by Region
5.3 Device Volume, by Region (2020-2032)
5.4 Healthcare Spending, by Region (Government, Commercial, Private, Out-of-Pocket) (2023)
6. Competitive Landscape
6.1 List of Major Companies, By Region
6.2 Market Share Analysis, By Region
6.3 Product Benchmarking
6.3.1 Product specifications and features
6.3.2 Pricing
6.4 Strategic Initiatives
6.4.1 Marketing and promotional activities
6.4.2 Distribution and Supply Chain Strategies
6.4.3 Expansion plans and new product launches
6.4.4 Strategic partnerships and collaborations
6.5 Technological Advancements
6.6 Market Positioning and Branding
7. Sleep Apnea Devices Market Segmentation, by Product
7.1 Chapter Overview
7.2 Therapeutic Devices
7.2.1 Therapeutic Devices Market Trends Analysis (2020-2032)
7.2.2 Therapeutic Devices Market Size Estimates and Forecasts to 2032 (USD Billion)
7.2.3 PAP Devices
7.2.3.1 PAP Devices Market Trends Analysis (2020-2032)
7.2.3.2 PAP Devices Market Size Estimates and Forecasts to 2032 (USD Billion)
7.2.3.3 CPAP Devices
7.2.3.3.1 CPAP Devices Market Trends Analysis (2020-2032)
7.2.3.3.2 CPAP Devices Market Size Estimates and Forecasts to 2032 (USD Billion)
7.2.3.4 APAP Devices
7.2.3.4.1 APAP Devices Market Trends Analysis (2020-2032)
7.2.3.4.2 APAP Devices Market Size Estimates and Forecasts to 2032 (USD Billion)
7.2.3.5 BPAP Devices
7.2.3.5.1 BPAP Devices Market Trends Analysis (2020-2032)
7.2.3.5.2 BPAP Devices Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.4 Facial Interfaces
7.3.4.1 Facial Interfaces Market Trends Analysis (2020-2032)
7.3.4.2 Facial Interfaces Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.4.3 Masks
7.3.4.3.1 Masks Market Trends Analysis (2020-2032)
7.3.4.3.2 Masks Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.4.3.3 Full-face Masks
7.3.4.3.3.1 Full-face Masks Market Trends Analysis (2020-2032)
7.3.4.3.3.2 Full-face Masks Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.4.3.4 Nasal Pillow Masks
7.3.4.3.4.1 Nasal Pillow Masks Market Trends Analysis (2020-2032)
7.3.4.3.4.2 Nasal Pillow Masks Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.4.3.5 Nasal Masks
7.3.4.3.5.1 Nasal Masks Market Trends Analysis (2020-2032)
7.3.4.3.5.2 Nasal Masks Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.5 Cushions
7.3.5.1 Cushions Market Trends Analysis (2020-2032)
7.3.5.2 Cushions Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.5 Accessories
7.3.5.1 Accessories Market Trends Analysis (2020-2032)
7.3.5.2 Accessories Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.5.3 Humidifiers Accessories
7.3.5.3.1 Humidifiers Accessories Market Trends Analysis (2020-2032)
7.3.5.3.2 Humidifiers Accessories Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.5.4 Power Accessories
7.3.5.4.1 Power Accessories Market Trends Analysis (2020-2032)
7.3.5.4.2 Power Accessories Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.5.5 Transportation Accessories
7.3.5.5.1 Transportation Accessories Market Trends Analysis (2020-2032)
7.3.5.5.2 Transportation Accessories Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.5.6 Communication Accessories
7.3.5.6.1 Communication Accessories Market Trends Analysis (2020-2032)
7.3.5.6.2 Communication Accessories Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.5.7 Chin Restraints
7.3.5.7.1 Chin Restraints Market Trends Analysis (2020-2032)
7.3.5.7.2 Chin Restraints Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.5.8 Other Accessories
7.3.5.8.1 Other Accessories Market Trends Analysis (2020-2032)
7.3.5.8.2 Other Accessories Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.6 Oral Appliances
7.3.6.1 Oral Appliances Market Trends Analysis (2020-2032)
7.3.6.2 Oral Appliances Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.6.3 Mandibular Advancement Devices
7.3.6.3.1 Mandibular Advancement Devices Market Trends Analysis (2020-2032)
7.3.6.3.2 Mandibular Advancement Devices Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.6.4 Tongue-retaining Devices
7.3.6.4.1 Tongue-retaining Devices Market Trends Analysis (2020-2032)
7.3.6.4.2 Tongue-retaining Devices Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.6.5 Daytime-Nighttime Appliances
7.3.6.5.1 Daytime-Nighttime Appliances Market Trends Analysis (2020-2032)
7.3.6.5.2 Daytime-Nighttime Appliances Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.7 Other Therapeutic Devices
7.3.7.1 Other Therapeutic Devices Market Trends Analysis (2020-2032)
7.3.7.2 Other Therapeutic Devices Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3 Diagnostic Devices
7.3.1 Diagnostic Devices Market Trends Analysis (2020-2032)
7.3.2 Diagnostic Devices Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.3 PSG Devices
7.3.3.1 PSG Devices Market Trends Analysis (2020-2032)
7.3.3.2 PSG Devices Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.3.3 Ambulatory PSG Devices
7.3.3.3.1 Ambulatory PSG Devices Market Trends Analysis (2020-2032)
7.3.3.3.2 Ambulatory PSG Devices Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.3.4 Clinical PSG Devices
7.3.3.4.1 Clinical PSG Devices Market Trends Analysis (2020-2032)
7.3.3.4.2 Clinical PSG Devices Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.4 Home Sleep Testing Devices
7.3.4.1 Home Sleep Testing Devices Market Trends Analysis (2020-2032)
7.3.4.2 Home Sleep Testing Devices Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.5 Oximeters
7.3.5.1 Oximeters Market Trends Analysis (2020-2032)
7.3.5.2 Oximeters Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.5.3 Fingertip Oximeters
7.3.5.3.1 Fingertip Oximeters Market Trends Analysis (2020-2032)
7.3.5.3.2 Fingertip Oximeters Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.5.4 Handheld Oximeters
7.3.5.4.1 Handheld Oximeters Market Trends Analysis (2020-2032)
7.3.5.4.2 Handheld Oximeters Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.5.5 Wrist-worn Oximeters
7.3.5.5.1 Wrist-worn Oximeters Market Trends Analysis (2020-2032)
7.3.5.5.2 Wrist-worn Oximeters Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.5.6 Tabletop Oximeters
7.3.5.6.1 Tabletop Oximeters Market Trends Analysis (2020-2032)
7.3.5.6.2 Tabletop Oximeters Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.6 Actigraphy Systems
7.3.6.1 Actigraphy Systems Market Trends Analysis (2020-2032)
7.3.6.2 Actigraphy Systems Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.7 Sleep Screening Devices
7.3.7.1 Sleep Screening Devices Market Trends Analysis (2020-2032)
7.3.7.2 Sleep Screening Devices Market Size Estimates and Forecasts to 2032 (USD Billion)
8. Sleep Apnea Devices Market Segmentation, By Age Group
8.1 Chapter Overview
8.2 Below 40 Years
8.2.1 Below 40 Years Market Trends Analysis (2020-2032)
8.2.2 Below 40 Years Market Size Estimates and Forecasts to 2032 (USD Billion)
8.3 40-60 Years
8.3.1 40-60 Years Market Trends Analysis (2020-2032)
8.3.2 40-60 Years Market Size Estimates And Forecasts To 2032 (USD Billion)
8.4 Above 60 Years
8.4.1 Above 60 Years Market Trends Analysis (2020-2032)
8.4.2 Above 60 Years Market Size Estimates And Forecasts To 2032 (USD Billion)
9. Sleep Apnea Devices Market Segmentation, by Gender
9.1 Chapter Overview
9.2 Male Patients
9.2.1 Male Patients Market Trends Analysis (2020-2032)
9.2.2 Male Patients Market Size Estimates and Forecasts to 2032 (USD Billion)
9.3 Female Patients
9.3.1 Female Patients Market Trends Analysis (2020-2032)
9.3.2 Female Patients Market Size Estimates and Forecasts to 2032 (USD Billion)
10. Sleep Apnea Devices Market Segmentation, By End User
10.1 Chapter Overview
10.2 Sleep Laboratories, Clinics, and Hospitals
10.2.1 Sleep Laboratories, Clinics, and Hospitals Market Trends Analysis (2020-2032)
10.2.2 Sleep Laboratories, Clinics, and Hospitals Market Size Estimates and Forecasts to 2032 (USD Billion)
10.3 Home Care Settings/ Individuals
10.3.1 Home Care Settings/ Individuals Market Trends Analysis (2020-2032)
10.3.2 Home Care Settings/ Individuals Market Size Estimates and Forecasts to 2032 (USD Billion)
11. Regional Analysis
11.1 Chapter Overview
11.2 North America
11.2.1 Trends Analysis
11.2.2 North America Sleep Apnea Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
11.2.3 North America Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.2.4 North America Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.2.5 North America Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.2.6 North America Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.2.7 USA
11.2.7.1 USA Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.2.7.2 USA Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.2.7.3 USA Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.2.7.4 USA Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.2.8 Canada
11.2.8.1 Canada Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.2.8.2 Canada Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.2.8.3 Canada Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.2.8.4 Canada Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.2.9 Mexico
11.2.9.1 Mexico Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.2.9.2 Mexico Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.2.9.3 Mexico Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.2.9.4 Mexico Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion
11.3 Europe
11.3.1 Eastern Europe
11.3.1.1 Trends Analysis
11.3.1.2 Eastern Europe Sleep Apnea Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
11.3.1.3 Eastern Europe Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.3.1.4 Eastern Europe Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.3.1.5 Eastern Europe Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.3.1.6 Eastern Europe Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.3.1.7 Poland
11.3.1.7.1 Poland Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.3.1.7.2 Poland Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.3.1.7.3 Poland Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.3.1.7.4 Poland Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.3.1.8 Romania
11.3.1.8.1 Romania Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.3.1.8.2 Romania Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.3.1.8.3 Romania Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.3.1.8.4 Romania Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.3.1.9 Hungary
11.3.1.9.1 Hungary Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.3.1.9.2 Hungary Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.3.1.9.3 Hungary Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.3.1.9.4 Hungary Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.3.1.10 Turkey
11.3.1.10.1 Turkey Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.3.1.10.2 Turkey Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.3.1.10.3 Turkey Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.3.1.10.4 Turkey Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.3.1.11 Rest of Eastern Europe
11.3.1.11.1 Rest of Eastern Europe Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.3.1.11.2 Rest of Eastern Europe Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.3.1.11.3 Rest of Eastern Europe Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.3.1.11.4 Rest of Eastern Europe Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.3.2 Western Europe
11.3.2.1 Trends Analysis
11.3.2.2 Western Europe Sleep Apnea Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
11.3.2.3 Western Europe Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.3.2.4 Western Europe Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.3.2.5 Western Europe Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.3.2.6 Western Europe Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.3.2.7 Germany
11.3.2.7.1 Germany Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.3.2.7.2 Germany Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.3.2.7.3 Germany Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.3.2.7.4 Germany Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.3.2.8 France
11.3.2.8.1 France Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.3.2.8.2 France Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.3.2.8.3 France Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.3.2.8.4 France Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.3.2.9 UK
11.3.2.9.1 UK Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.3.2.9.2 UK Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.3.2.9.3 UK Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.3.2.9.4 UK Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.3.2.10 Italy
11.3.2.10.1 Italy Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.3.2.10.2 Italy Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.3.2.10.3 Italy Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.3.2.10.4 Italy Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.3.2.11 Spain
11.3.2.11.1 Spain Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.3.2.11.2 Spain Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.3.2.11.3 Spain Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.3.2.11.4 Spain Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.3.2.12 Netherlands
11.3.2.12.1 Netherlands Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.3.2.12.2 Netherlands Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.3.2.12.3 Netherlands Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.3.2.12.4 Netherlands Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.3.2.13 Switzerland
11.3.2.13.1 Switzerland Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.3.2.13.2 Switzerland Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.3.2.13.3 Switzerland Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.3.2.13.4 Switzerland Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.3.2.14 Austria
11.3.2.14.1 Austria Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.3.2.14.2 Austria Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.3.2.14.3 Austria Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.3.2.14.4 Austria Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.3.2.15 Rest of Western Europe
11.3.2.15.1 Rest of Western Europe Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.3.2.15.2 Rest of Western Europe Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.3.2.15.3 Rest of Western Europe Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.3.2.15.4 Rest of Western Europe Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.4 Asia Pacific
11.4.1 Trends Analysis
11.4.2 Asia Pacific Sleep Apnea Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
11.4.3 Asia Pacific Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.4.4 Asia Pacific Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.4.5 Asia Pacific Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.4.6 Asia Pacific Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.4.7 China
11.4.7.1 China Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.4.7.2 China Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.4.7.3 China Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.4.7.4 China Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.4.8 India
11.4.8.1 India Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.4.8.2 India Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.4.8.3 India Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.4.8.4 India Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.4.9 Japan
11.4.9.1 Japan Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.4.9.2 Japan Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.4.9.3 Japan Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.4.9.4 Japan Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.4.10 South Korea
11.4.10.1 South Korea Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.4.10.2 South Korea Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.4.10.3 South Korea Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.4.10.4 South Korea Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.4.11 Vietnam
11.4.11.1 Vietnam Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.4.11.2 Vietnam Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.4.11.3 Vietnam Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.4.11.4 Vietnam Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.4.12 Singapore
11.4.12.1 Singapore Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.4.12.2 Singapore Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.4.12.3 Singapore Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.4.12.4 Singapore Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.4.13 Australia
11.4.13.1 Australia Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.4.13.2 Australia Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.4.13.3 Australia Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.4.13.4 Australia Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.4.14 Rest of Asia Pacific
11.4.14.1 Rest of Asia Pacific Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.4.14.2 Rest of Asia Pacific Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.4.14.3 Rest of Asia Pacific Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.4.14.4 Rest of Asia Pacific Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.5 Middle East and Africa
11.5.1 Middle East
11.5.1.1 Trends Analysis
11.5.1.2 Middle East Sleep Apnea Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
11.5.1.3 Middle East Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.5.1.4 Middle East Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.5.1.5 Middle East Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.5.1.6 Middle East Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.5.1.7 UAE
11.5.1.7.1 UAE Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.5.1.7.2 UAE Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.5.1.7.3 UAE Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.5.1.7.4 UAE Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.5.1.8 Egypt
11.5.1.8.1 Egypt Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.5.1.8.2 Egypt Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.5.1.8.3 Egypt Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.5.1.8.4 Egypt Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.5.1.9 Saudi Arabia
11.5.1.9.1 Saudi Arabia Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.5.1.9.2 Saudi Arabia Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.5.1.9.3 Saudi Arabia Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.5.1.9.4 Saudi Arabia Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.5.1.10 Qatar
11.5.1.10.1 Qatar Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.5.1.10.2 Qatar Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.5.1.10.3 Qatar Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.5.1.10.4 Qatar Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.5.1.11 Rest of Middle East
11.5.1.11.1 Rest of Middle East Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.5.1.11.2 Rest of Middle East Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.5.1.11.3 Rest of Middle East Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.5.1.11.4 Rest of Middle East Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.5.2 Africa
11.5.2.1 Trends Analysis
11.5.2.2 Africa Sleep Apnea Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
11.5.2.3 Africa Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.5.2.4 Africa Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.5.2.5 Africa Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.5.2.6 Africa Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.5.2.7 South Africa
11.5.2.7.1 South Africa Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.5.2.7.2 South Africa Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.5.2.7.3 South Africa Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.5.2.7.4 South Africa Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.5.2.8 Nigeria
11.5.2.8.1 Nigeria Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.5.2.8.2 Nigeria Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.5.2.8.3 Nigeria Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.5.2.8.4 Nigeria Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.5.2.9 Rest of Africa
11.5.2.9.1 Rest of Africa Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.5.2.9.2 Rest of Africa Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.5.2.9.3 Rest of Africa Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.5.2.9.4 Rest of Africa Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.6 Latin America
11.6.1 Trends Analysis
11.6.2 Latin America Sleep Apnea Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
11.6.3 Latin America Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.6.4 Latin America Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.6.5 Latin America Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.6.6 Latin America Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.6.7 Brazil
11.6.7.1 Brazil Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.6.7.2 Brazil Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.6.7.3 Brazil Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.6.7.4 Brazil Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.6.8 Argentina
11.6.8.1 Argentina Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.6.8.2 Argentina Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.6.8.3 Argentina Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.6.8.4 Argentina Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.6.9 Colombia
11.6.9.1 Colombia Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.6.9.2 Colombia Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.6.9.3 Colombia Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.6.9.4 Colombia Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
11.6.10 Rest of Latin America
11.6.10.1 Rest of Latin America Sleep Apnea Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.6.10.2 Rest of Latin America Sleep Apnea Devices Market Estimates and Forecasts, By Age Group (2020-2032) (USD Billion)
11.6.10.3 Rest of Latin America Sleep Apnea Devices Market Estimates and Forecasts, by Gender (2020-2032) (USD Billion)
11.6.10.4 Rest of Latin America Sleep Apnea Devices Market Estimates and Forecasts, By End User (2020-2032) (USD Billion)
12. Company Profiles
12.1 Respironics (a subsidiary of Koninklijke Philips N.V.)
12.1.1 Company Overview
12.1.2 Financial
12.1.3 Products/ Services Offered
12.1.4 SWOT Analysis
12.2 ResMed
12.2.1 Company Overview
12.2.2 Financial
12.2.3 Products/ Services Offered
12.2.4 SWOT Analysis
12.3 Fisher & Paykel Healthcare Limited
12.3.1 Company Overview
12.3.2 Financial
12.3.3 Products/ Services Offered
12.3.4 SWOT Analysis
12.4 Curative Medical, Inc.
12.4.1 Company Overview
12.4.2 Financial
12.4.3 Products/ Services Offered
12.4.4 SWOT Analysis
12.5 React Health (Respiratory Product Line from Invacare Corporation)
12.5.1 Company Overview
12.5.2 Financial
12.5.3 Products/ Services Offered
12.5.4 SWOT Analysis
12.6 Somnetics International, Inc.
12.6.1 Company Overview
12.6.2 Financial
12.6.3 Products/ Services Offered
12.6.4 SWOT Analysis
12.7 BMC Medical Co., Ltd.
12.7.1 Company Overview
12.7.2 Financial
12.7.3 Products/ Services Offered
12.7.4 SWOT Analysis
12.8 Natus Medical Incorporated
12.8.1 Company Overview
12.8.2 Financial
12.8.3 Products/ Services Offered
12.8.4 SWOT Analysis
12.9 Nihon Kohden Corporation
12.9.1 Company Overview
12.9.2 Financial
12.9.3 Products/ Services Offered
12.9.4 SWOT Analysis
12.10 Cadwell Industries, Inc.
12.10.1 Company Overview
12.10.2 Financial
12.10.3 Products/ Services Offered
12.10.4 SWOT Analysis
13. Use Cases and Best Practices
14. Conclusion
An accurate research report requires proper strategizing as well as implementation. There are multiple factors involved in the completion of good and accurate research report and selecting the best methodology to compete the research is the toughest part. Since the research reports we provide play a crucial role in any company’s decision-making process, therefore we at SNS Insider always believe that we should choose the best method which gives us results closer to reality. This allows us to reach at a stage wherein we can provide our clients best and accurate investment to output ratio.
Each report that we prepare takes a timeframe of 350-400 business hours for production. Starting from the selection of titles through a couple of in-depth brain storming session to the final QC process before uploading our titles on our website we dedicate around 350 working hours. The titles are selected based on their current market cap and the foreseen CAGR and growth.
The 5 steps process:
Step 1: Secondary Research:
Secondary Research or Desk Research is as the name suggests is a research process wherein, we collect data through the readily available information. In this process we use various paid and unpaid databases which our team has access to and gather data through the same. This includes examining of listed companies’ annual reports, Journals, SEC filling etc. Apart from this our team has access to various associations across the globe across different industries. Lastly, we have exchange relationships with various university as well as individual libraries.

Step 2: Primary Research
When we talk about primary research, it is a type of study in which the researchers collect relevant data samples directly, rather than relying on previously collected data. This type of research is focused on gaining content specific facts that can be sued to solve specific problems. Since the collected data is fresh and first hand therefore it makes the study more accurate and genuine.
We at SNS Insider have divided Primary Research into 2 parts.
Part 1 wherein we interview the KOLs of major players as well as the upcoming ones across various geographic regions. This allows us to have their view over the market scenario and acts as an important tool to come closer to the accurate market numbers. As many as 45 paid and unpaid primary interviews are taken from both the demand and supply side of the industry to make sure we land at an accurate judgement and analysis of the market.
This step involves the triangulation of data wherein our team analyses the interview transcripts, online survey responses and observation of on filed participants. The below mentioned chart should give a better understanding of the part 1 of the primary interview.

Part 2: In this part of primary research the data collected via secondary research and the part 1 of the primary research is validated with the interviews from individual consultants and subject matter experts.
Consultants are those set of people who have at least 12 years of experience and expertise within the industry whereas Subject Matter Experts are those with at least 15 years of experience behind their back within the same space. The data with the help of two main processes i.e., FGDs (Focused Group Discussions) and IDs (Individual Discussions). This gives us a 3rd party nonbiased primary view of the market scenario making it a more dependable one while collation of the data pointers.
Step 3: Data Bank Validation
Once all the information is collected via primary and secondary sources, we run that information for data validation. At our intelligence centre our research heads track a lot of information related to the market which includes the quarterly reports, the daily stock prices, and other relevant information. Our data bank server gets updated every fortnight and that is how the information which we collected using our primary and secondary information is revalidated in real time.

Step 4: QA/QC Process
After all the data collection and validation our team does a final level of quality check and quality assurance to get rid of any unwanted or undesired mistakes. This might include but not limited to getting rid of the any typos, duplication of numbers or missing of any important information. The people involved in this process include technical content writers, research heads and graphics people. Once this process is completed the title gets uploader on our platform for our clients to read it.
Step 5: Final QC/QA Process:
This is the last process and comes when the client has ordered the study. In this process a final QA/QC is done before the study is emailed to the client. Since we believe in giving our clients a good experience of our research studies, therefore, to make sure that we do not lack at our end in any way humanly possible we do a final round of quality check and then dispatch the study to the client.
By Product
Therapeutic Devices
PAP Devices
CPAP Devices
APAP Devices
BPAP Devices
Facial Interfaces
Masks
Full-face Masks
Nasal Pillow Masks
Nasal Masks
Cushions
Accessories
Humidifiers Accessories
Power Accessories
Transportation Accessories
Communication Accessories
Chin Restraints
Other Accessories
Oral Appliances
Mandibular Advancement Devices
Tongue-retaining Devices
Daytime-Nighttime Appliances
Other Therapeutic Devices
Diagnostic Devices
PSG Devices
Ambulatory PSG Devices
Clinical PSG Devices
Home Sleep Testing Devices
Oximeters
Fingertip Oximeters
Handheld Oximeters
Wrist-worn Oximeters
Tabletop Oximeters
Actigraphy Systems
Sleep Screening Devices
By Age Group
Below 40 Years
40-60 Years
Above 60 Years
By Gender
Male Patients
Female Patients
By End User
Sleep Laboratories, Clinics, and Hospitals
Home Care Settings/ Individuals
Request for Segment Customization as per your Business Requirement: Segment Customization Request
Regional Coverage:
North America
US
Canada
Mexico
Europe
Eastern Europe
Poland
Romania
Hungary
Turkey
Rest of Eastern Europe
Western Europe
Germany
France
UK
Italy
Spain
Netherlands
Switzerland
Austria
Rest of Western Europe
Asia Pacific
China
India
Japan
South Korea
Vietnam
Singapore
Australia
Rest of Asia Pacific
Middle East & Africa
Middle East
UAE
Egypt
Saudi Arabia
Qatar
Rest of the Middle East
Africa
Nigeria
South Africa
Rest of Africa
Latin America
Brazil
Argentina
Colombia
Rest of Latin America
Request for Country Level Research Report: Country Level Customization Request
Available Customization
With the given market data, SNS Insider offers customization as per the company’s specific needs. The following customization options are available for the report:
Product Analysis
Criss-Cross segment analysis (e.g. Product X Application)
Product Matrix which gives a detailed comparison of the product portfolio of each company
Geographic Analysis
Additional countries in any of the regions
Company Information
Detailed analysis and profiling of additional market players (Up to five)
The Cancer Stem Cells Market Size was USD 4.06 billion in 2023 and is expected to reach USD 8.41 billion by 2032, growing at a CAGR of 8.43% by 2024-2032.
The Population Screening Market was valued at USD 26.65 billion in 2023 and is expected to reach USD 42.48 billion by 2032, growing at a CAGR of 5.34% over the forecast period 2024-2032.
The Healthcare IT Consulting Market Size was valued at USD 50.1 billion in 2023 and is expected to reach USD 168.14 billion by 2032 and grow at a CAGR of 14.4% over the forecast period 2024-2032.
The Blood Pressure Monitors Market size was valued at USD 1.81 Billion in 2023 and is estimated to grow to USD 4.28 Billion by 2032, with a 10.04% CAGR.
The Inflammatory Bowel Disease Drugs Market size was valued at USD 21.15 Billion in 2023 and is expected to reach USD 27.65 Billion By 2031 and grow at a CAGR of 3.47% over the forecast period of 2024-2031.
The Immunoprotein Diagnostic Testing Market size was valued at USD 8.89 billion in 2023, expected to reach USD 17.13 billion by 2032, growing at a 7.54%.
Hi! Click one of our member below to chat on Phone