The Risk-based Monitoring Software Market Size was valued at USD 359.32 Million in 2023, and is expected to reach USD 1004.32 Million by 2031, and grow at a CAGR of 13.71% over the forecast period 2024-2031.
Risk-based monitoring software is a type of clinical trial monitoring software that complies with regulatory criteria. It does, nevertheless, move away from patient data source data verification (SDV). It uses numerous platforms, tools, and dashboards to identify signals that are used to solve issues connected to clinical trial safety, data integrity, and compliance. This risk-based monitoring program aids in the emphasis on trials without putting high-value tasks at risk. The growing number of clinical trials, as well as the increase in government funding and grants for clinical trials, all contribute to the growth of the RBM software market. At the time of speculation, however, the high cost of implementing RBM programs is expected to slow market growth.
Get more information on Risk-based Monitoring Software Market - Request Sample Report
DRIVERS
RBM systems are cost- and time-effective.
Clinical studies are on the rise.
Grants and financing from the government to support clinical trials are being increased.
RESTRAINTS
Implementation expenses are high.
OPPORTUNITIES
Clinical trial processes are increasingly being outsourced to contract research organisations (CROs).
CHALLENGES
Scarcity of qualified personnel to operate RBM solutions
IMPACT OF COVID-19
Medical supplies are in high demand in order to care for the sick populace. Among the most commonly utilised medical equipment in primary clinical care are atomizers, life-support machines, oxygen generators, and monitors. Furthermore, COVID-19 has resulted in a significant increase in demand for medical goods such as masks, gloves, and eye protection. As the number of COVID-19 cases increases around the world, the demand for medical supplies from both healthcare professionals and the general public grows.
By Type
The enterprise and site RBM software markets are divided into two categories. In comparison to the type of site, the enterprise is the largest segment. This software allows academics and professionals to obtain clinical test results from a single location, resulting in high demand from end-users.
By Component
RBM software is divided into two categories: software and services. The RBM software market is likely to be dominated by the software segment. The expanding R&D expenditure in the life science and clinical research industries, as well as an increasing number of clinical trials and a growing client base, can be attributable to this segment's high proportion.
By Delivery Mode
The RBM software market is divided into two categories: Business Licensed (On-premise) and Cloud-based (SaaS). The web-based segment (Wanted) is expected to have a strong presence in the RBM software market. The benefits of web-based software, such as easy access, improved productivity, time efficiency, and cost effectiveness, make up the bulk of this category.
By End-User
Pharmaceutical and biopharmaceutical firms, CROs, medical equipment companies, and other end users are building the RBM software industry. The pharmaceutical and biopharmaceutical industry segment is predicting that it will dominate the RBM software market. An important factor driving the growth of this consumer market is the use of costly R&D for pharmaceutical and biopharmaceutical businesses.
By Type
Site RBM Software
Enterprise RBM Software
By Component
Services
Software
By Delivery Mode
Licensed Enterprise (On-premise)
Cloud-based (SaaS)
By End-User
Pharmaceutical & Biopharmaceutical Companies
Medical Device Companies
CROs
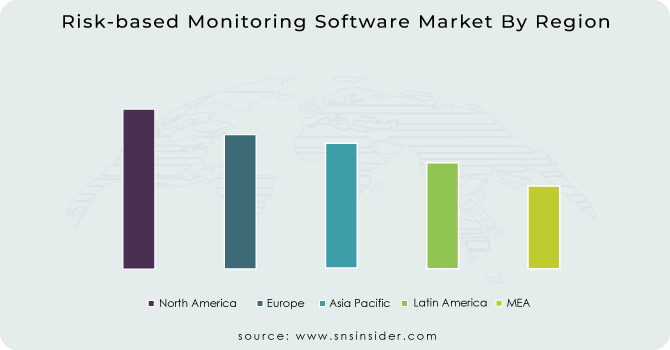
RBM software market in North America is estimated to account for the highest share of the global market. At the time of speculation, the Asia Pacific market was predicted to grow at a rapid pace. This increase may be due to increased government funding for clinical trials, stronger control guidelines than developed countries, greater patient base, lower operating costs for clinical trials, a shortage of volunteers in Europe and North America, and a growing number. of pharmaceutical companies and contract research organizations (CROs) in the region.
Need any customization research on Risk-based Monitoring Software Market - Enquiry Now
REGIONAL COVERAGE:
North America
USA
Canada
Mexico
Europe
Germany
UK
France
Italy
Spain
The Netherlands
Rest of Europe
Asia-Pacific
Japan
south Korea
China
India
Australia
Rest of Asia-Pacific
The Middle East & Africa
Israel
UAE
South Africa
Rest of Middle East & Africa
Latin America
Brazil
Argentina
Rest of Latin America
Some of the major key players are as follows: ArisGlobal, Anju Software, Bioclinica, DATATRAK, Forte Research Systems, MedNet Solutions, IBM Corporation, Medidata Solutions, Oracle, Parexe and Other Players.
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | US$ 359.32 Million |
| Market Size by 2031 | US$ 1004.32 Million |
| CAGR | CAGR of 13.71% From 2024 to 2031 |
| Base Year | 2023 |
| Forecast Period | 2024-2031 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Type (Site RBM Software, Enterprise RBM Software) • By Component (Services, Software) • By Delivery Mode (Licensed Enterprise (On-premise), Cloud-based (SaaS)) • By End-User (Pharmaceutical & Biopharmaceutical Companies, Medical Device Companies, CROs) |
| Regional Analysis/Coverage | North America (USA, Canada, Mexico), Europe (Germany, UK, France, Italy, Spain, Netherlands, Rest of Europe), Asia-Pacific (Japan, South Korea, China, India, Australia, Rest of Asia-Pacific), The Middle East & Africa (Israel, UAE, South Africa, Rest of Middle East & Africa), Latin America (Brazil, Argentina, Rest of Latin America) |
| Company Profiles | ArisGlobal, Anju Software, Bioclinica, DATATRAK, Forte Research Systems, MedNet Solutions, IBM Corporation, Medidata Solutions, Oracle, Parexel. |
| DRIVERS | • RBM systems are cost- and time-effective. • Clinical studies are on the rise. • Grants and financing from the government to support clinical trials are being increased. |
| RESTRAINTS | • Implementation expenses are high. |
Risk-based Monitoring Software Market Size was valued at USD 359.32 Million in 2023.
RBM systems are cost- and time-effective, Clinical studies are on the rise are the key drivers of the Risk-based Monitoring Software market
RBM software market in North America is estimated to account for the highest share of the global market.
Manufacturer and service provider, research institutes, university and private libraries, suppliers and distributers.
The risk-based Monitoring Software market is divided into four segments they are By Type, By Component, By Delivery Mode, and By End-User.
Table of Contents
1. Introduction
1.1 Market Definition
1.2 Scope
1.3 Research Assumptions
2. Research Methodology
3. Market Dynamics
3.1 Drivers
3.2 Restraints
3.3 Opportunities
3.4 Challenges
4. Impact Analysis
4.1 COVID-19 Impact Analysis
4.2 Impact of Ukraine- Russia war
4.3 Impact of ongoing Recession
4.3.1 Introduction
4.3.2 Impact on major economies
4.3.2.1 US
4.3.2.2 Canada
4.3.2.3 Germany
4.3.2.4 France
4.3.2.5 United Kingdom
4.3.2.6 China
4.3.2.7 Japan
4.3.2.8 South Korea
4.3.2.9 Rest of the World
5. Value Chain Analysis
6. Porter’s 5 forces model
7. PEST Analysis
8. Risk-based Monitoring Software Market Segmentation, By Type
8.1 Site RBM Software
8.2 Enterprise RBM Software
9. Risk-based Monitoring Software Market Segmentation, By Component
9.1 Services
9.2 Software
10. Risk-based Monitoring Software Market Segmentation, By Delivery Mode
10.1 Licensed Enterprise (On-premise)
10.2 Cloud-based (SaaS)
11. Risk-based Monitoring Software Market Segmentation, By End-User
11.1 Pharmaceutical & Biopharmaceutical Companies
11.2 Medical Device Companies
11.3 CROs
12. Regional Analysis
12.1 Introduction
12.2 North America
12.2.1 USA
12.2.2 Canada
12.2.3 Mexico
12.3 Europe
12.3.1 Germany
12.3.2 UK
12.3.3 France
12.3.4 Italy
12.3.5 Spain
12.3.6 The Netherlands
12.3.7 Rest of Europe
12.4 Asia-Pacific
12.4.1 Japan
12.4.2 South Korea
12.4.3 China
12.4.4 India
12.4.5 Australia
12.4.6 Rest of Asia-Pacific
12.5 The Middle East & Africa
12.5.1 Israel
12.5.2 UAE
12.5.3 South Africa
12.5.4 Rest
12.6 Latin America
12.6.1 Brazil
12.6.2 Argentina
12.6.3 Rest of Latin America
13. Company Profiles
13.1 Anju Software
13.1.1 Financial
13.1.2 Products/ Services Offered
13.1.3 SWOT Analysis
13.1.4 The SNS view
13.2 ArisGlobal
13.3 Bioclinica, DATATRAK
13.4 Forte Research Systems
13.5 MedNet Solutions
13.6 IBM Corporation
13.7 Medidata Solutions
13.8 Oracle
13.9 Parexel.
14. Competitive Landscape
14.1 Competitive Benchmark
14.2 Market Share Analysis
14.3 Recent Developments
15. Conclusion
An accurate research report requires proper strategizing as well as implementation. There are multiple factors involved in the completion of good and accurate research report and selecting the best methodology to compete the research is the toughest part. Since the research reports we provide play a crucial role in any company’s decision-making process, therefore we at SNS Insider always believe that we should choose the best method which gives us results closer to reality. This allows us to reach at a stage wherein we can provide our clients best and accurate investment to output ratio.
Each report that we prepare takes a timeframe of 350-400 business hours for production. Starting from the selection of titles through a couple of in-depth brain storming session to the final QC process before uploading our titles on our website we dedicate around 350 working hours. The titles are selected based on their current market cap and the foreseen CAGR and growth.
The 5 steps process:
Step 1: Secondary Research:
Secondary Research or Desk Research is as the name suggests is a research process wherein, we collect data through the readily available information. In this process we use various paid and unpaid databases which our team has access to and gather data through the same. This includes examining of listed companies’ annual reports, Journals, SEC filling etc. Apart from this our team has access to various associations across the globe across different industries. Lastly, we have exchange relationships with various university as well as individual libraries.

Step 2: Primary Research
When we talk about primary research, it is a type of study in which the researchers collect relevant data samples directly, rather than relying on previously collected data. This type of research is focused on gaining content specific facts that can be sued to solve specific problems. Since the collected data is fresh and first hand therefore it makes the study more accurate and genuine.
We at SNS Insider have divided Primary Research into 2 parts.
Part 1 wherein we interview the KOLs of major players as well as the upcoming ones across various geographic regions. This allows us to have their view over the market scenario and acts as an important tool to come closer to the accurate market numbers. As many as 45 paid and unpaid primary interviews are taken from both the demand and supply side of the industry to make sure we land at an accurate judgement and analysis of the market.
This step involves the triangulation of data wherein our team analyses the interview transcripts, online survey responses and observation of on filed participants. The below mentioned chart should give a better understanding of the part 1 of the primary interview.

Part 2: In this part of primary research the data collected via secondary research and the part 1 of the primary research is validated with the interviews from individual consultants and subject matter experts.
Consultants are those set of people who have at least 12 years of experience and expertise within the industry whereas Subject Matter Experts are those with at least 15 years of experience behind their back within the same space. The data with the help of two main processes i.e., FGDs (Focused Group Discussions) and IDs (Individual Discussions). This gives us a 3rd party nonbiased primary view of the market scenario making it a more dependable one while collation of the data pointers.
Step 3: Data Bank Validation
Once all the information is collected via primary and secondary sources, we run that information for data validation. At our intelligence centre our research heads track a lot of information related to the market which includes the quarterly reports, the daily stock prices, and other relevant information. Our data bank server gets updated every fortnight and that is how the information which we collected using our primary and secondary information is revalidated in real time.

Step 4: QA/QC Process
After all the data collection and validation our team does a final level of quality check and quality assurance to get rid of any unwanted or undesired mistakes. This might include but not limited to getting rid of the any typos, duplication of numbers or missing of any important information. The people involved in this process include technical content writers, research heads and graphics people. Once this process is completed the title gets uploader on our platform for our clients to read it.
Step 5: Final QC/QA Process:
This is the last process and comes when the client has ordered the study. In this process a final QA/QC is done before the study is emailed to the client. Since we believe in giving our clients a good experience of our research studies, therefore, to make sure that we do not lack at our end in any way humanly possible we do a final round of quality check and then dispatch the study to the client.
The Military Wearable Medical Device Market Size was valued at USD 7.84 billion in 2023 and is expected to reach USD 64.47 billion by 2032, and grow at a CAGR of 26.40% over the forecast period 2024-2032.
The Physical Therapy Software Market was valued at USD 1.19 billion in 2023 and is expected to reach USD 2.94 billion by 2032, growing at a CAGR of 10.47% from 2024-2032.
The global blood testing market was valued at USD 91.43 Bn in 2023 and is expected to reach USD 169.15 Bn by 2032, growing at a CAGR of 7.08% from 2024 to 2032.
The Rapid Tests Market was valued at USD 1.46 billion in 2023 and is expected to reach USD 3.08 billion by 2032, with a CAGR of 8.64% from 2024 to 2032.
Pharmaceutical Sterility Testing Market was valued at USD 1.42 billion in 2023 and is expected to reach USD 3.79 billion by 2032, growing at a CAGR of 11.58%.
The Pharmaceutical Filtration Market Size was valued at USD 16.4 billion in 2023 and is expected to reach USD 43.9 billion by 2032 with a growing CAGR of 11.56 % over the forecast period 2024-2032.
Hi! Click one of our member below to chat on Phone