The Rare Disease Genetic Testing Market was valued at USD 1.05 billion in 2023 and is expected to reach USD 3.79 billion by 2032, growing at a CAGR of 15.36% over the forecast period of 2024-2032. This report identifies the incidence and prevalence of rare diseases as driving demand for early and correct diagnostic solutions through increased demand. The research considers trends in the utilization of genetic testing by regions based on improvements in sequencing technologies as well as widening newborn screening programs. It further investigates healthcare expenditure on rare disease genetic testing with differences in the sources of funds, such as government programs, private insurance, and out-of-pocket spending affecting access. The report touches on technological breakthroughs, such as next-generation sequencing (NGS) and analytics powered by artificial intelligence (AI), that enhance diagnostic efficacy and decrease turnaround times. Regulation and policy patterns drive market development, with governments introducing frameworks that advance test standardization and reimbursement. Market penetration issues and accessibility impediments are further examined, focusing on inequalities regarding the availability of tests across different healthcare systems. In addition, patient and physician awareness patterns are changing, with more education efforts enhancing early diagnosis and treatment rates.
The U.S. Rare Disease Genetic Testing Market was valued at USD 0.31 billion in 2023 and is expected to reach USD 1.18 billion by 2032, growing at a CAGR of 16.01% over the forecast period of 2024-2032. In the United States, the rare disease genetic testing market is growing as a result of robust federal initiatives, increasing precision medicine investments, and enhanced collaborations between biotech companies and research organizations to improve early detection and tailored treatment approaches.
Drivers
The increasing prevalence of rare diseases is a major driver for the rare disease genetic testing market, with over 300 million people worldwide affected by these conditions.
Early and accurate diagnosis is crucial since nearly 80% of rare diseases have a genetic origin. The increasing uptake of Next-Generation Sequencing (NGS) has transformed diagnostics by allowing for rapid, cost-efficient, and high-throughput genetic testing. Advances in technology, including AI-based genomic analysis, have further improved diagnostic accuracy. Government support and research funding have played a major role in supporting market growth. For instance, initiatives such as the Undiagnosed Diseases Network (UDN) in the United States and Genomics England's 100,000 Genomes Project have enhanced access to genetic testing. Expanded newborn screening programs for inherited metabolic diseases also support market growth. Moreover, increased Direct-to-Consumer (DTC) genetic testing has promoted genetic screening, increasing awareness and early detection of diseases. The increase in pharma partnerships for precision medicine and orphan drug development also underlines market growth. Biopharmaceutical firms increasingly count on genetic tests to determine patient populations for drugs with a precise target, driving demand. The declining price of genetic sequencing—from USD 100 million in 2001 to below USD 600 in 2023—has also stimulated adoption in healthcare environments.
Restraints
The market faces significant restraints, primarily due to high costs and reimbursement limitations.
Even with the reduction in sequencing prices, full genetic testing can still cost between USD 1,000 and USD 5,000 and is thus expensive. Insurers only cover genetic tests for a given disease in most cases, and this restricts patient access. Ethical issues and privacy around the storage and sharing of genetic information are also limiting factors. Based on the Global Alliance for Genomics and Health (GA4GH), a shortage of regulated data-sharing policies limits the promise of large-scale genomic studies. Furthermore, there is a shortage of awareness and trained personnel that limits large-scale adoption. Most healthcare professionals are not trained to analyze complex genetic test reports, which causes delays in diagnoses. In developing countries, a shortage of genomic infrastructure and specialized laboratories limits access. Lack of standardized regulatory systems across regions also makes it difficult to expand markets. Though programs such as the EU's Orphan Drug Regulation promote research into rare diseases, the intricate process of regulatory approval for genetic tests postpones commercialization. In addition, high rates of false positives and misdiagnosis caused by inadequate variant databases undermine market credibility. To overcome these constraints, policy changes, improved reimbursement schemes, and investments in genomic training are necessary.
Opportunities
The rare disease genetic testing market presents substantial opportunities, particularly in personalized medicine and precision therapeutics.
Technological leaps in gene therapy and CRISPR-based diagnostics are creating new opportunities for precision treatments. The growth of whole genome sequencing (WGS) programs in national healthcare systems, for example, China's 1 Million Genomes Project, offers enormous expansion opportunities. The growing implementation of AI and machine learning in genetics analysis improves diagnosis accuracy, enhancing clinical decision-making. Partnerships between biotechnology companies and genetic testing companies are speeding up rare disease biomarker discovery, hence quicker diagnosis and treatment development. Growth in newborn screening programs, particularly in Latin America and the Asia-Pacific regions, is yet another key growth driver. Growing Direct-to-Consumer (DTC) genetic testing is offering an untapped market segment opportunity, with vendors such as Color Genomics and 23andMe increasing the scope of their rare disease tests. Moreover, telehealth-delivered genetic counseling is enhancing access and overcoming geographical constraints. Greater electronic health record (EHR) integration with genetic databases is simplifying test interpretation and tailored treatment advice. The trend toward universal genomic data-sharing platforms, including the Global Variome Project, seeks to enhance genetic variant interpretation, opening new doors for rare disease diagnosis and drug discovery.
Challenges
The market faces several challenges, primarily due to complex regulatory hurdles and data standardization issues.
Unlike other diagnostic tests, genetic testing must adhere to strict regulations such as CLIA, CAP, and IVDR in various markets, which poses challenges to entering global markets. There is no unified classification of genetic variants in databases, causing test interpretation inconsistencies. In the American Journal of Human Genetics study, more than 25% of genetic variants are misclassified, posing a risk of misdiagnosis. Another challenge lies with patient unwillingness due to fears of genetic discrimination despite laws such as the Genetic Information Nondiscrimination Act (GINA). Lack of large amounts of diverse genetic databases leads to diminished diagnostic performance among underrepresented populations. Infrastructural restraints in low-income areas prohibit universal use of genetic testing. Cybersecurity and data privacy are another pressing concern, with over 60 million health records hacked in 2023, raising the risk of genomic data compromise. Furthermore, the very high rate of failure for rare disease drug development, as almost 90% of orphan drug trials fail, undermines investment confidence in genetic diagnostics. These challenges need to be overcome through international regulatory harmonization, enhanced genomic data diversity, strengthened cybersecurity frameworks, and better genetic education programs.
By Disease
In 2023, the segment of immunological disorders led the rare disease genetic testing market, with the highest share of 10.5%. This leadership was fueled by the growing incidence of autoimmune and immune deficiency diseases, along with advances in genetic diagnostics enhancing early diagnosis. Government programs and increasing investments in immunogenetics research also helped boost market growth.
Conversely, the endocrine & metabolism diseases category proved to be the fastest-growing segment. The demand was fueled by an elevated number of inherited metabolic disorders, coupled with increased awareness and accessibility of genetic testing. The growth of newborn screening programs for metabolic disease caused the demand for genetic testing in this segment to increase.
By Technology
The Next-Generation Sequencing (NGS) segment was the market leader in 2023 with the highest share of 33.9%. NGS was driven by its throughput, cost-effectiveness, and capacity to analyze multiple genetic variations at once. Its uptake was also boosted by the decreasing price of sequencing and advances in bioinformatics for diagnosing rare diseases.
By Specialty
The segment of molecular genetic tests led the rare disease genetic testing market in 2023 with the highest revenue contribution of 38.2%. The leadership of this segment was due to its high precision in detecting single-gene mutations and hereditary disorders. Increasing demand for molecular methods, such as whole exome and whole genome sequencing, also contributed to market share.
By End Use
Among end users, research laboratories & CROs were the dominant market in 2023, with a share of 43.6%. This was mainly because of the growing emphasis on research related to rare diseases, growth in the number of clinical trials, and collaboration between pharmaceutical companies and genetic testing service providers. The need for sophisticated genetic analysis in drug discovery and personalized medicine played a crucial role in dominating this segment.
The diagnostic laboratories category was recognized as the most rapidly growing segment. The growing incorporation of genetic testing within regular clinical diagnostics, along with enhanced access to genetic counseling, fueled this expansion. Increased patient awareness and support for rare disease diagnosis by regulation also played an essential part in widening this segment.
North America held the largest share in the rare disease genetic testing market in 2023, fueled by sophisticated healthcare infrastructure, high usage of genetic testing, and robust government support. The U.S. dominates the market because of programs like the All of Us Research Program, which seeks to sequence one million genomes for precision medicine. The availability of leading genetic testing firms, such as Invitae, Illumina, and Natera, has also increased market growth. In addition, the National Institutes of Health (NIH) and Undiagnosed Diseases Network (UDN) are increasing access to genetic diagnostics, speeding up early disease detection.
Europe is next as a leading market because of the EU's Orphan Drug Regulation and high-volume sequencing initiatives such as Genomics England's 100,000 Genomes Project. Germany, France, and the UK are investing in incorporating genomic medicine into national healthcare systems, fueling demand.
The Asia-Pacific region is the most rapidly growing, fueled by growing adoption of genetic testing, expanding healthcare investments, and huge patient bases. China's 1 Million Genomes Project and India's Genome India Initiative are revolutionizing the landscape. Japan's USD 1.3 billion investment in rare disease research has further fueled growth. With growing newborn screening programs and increasing awareness of genetic disorders, the Asia-Pacific market is likely to experience the highest growth rate in the coming years.
Quest Diagnostics Inc. – Neurome, GeneDx, ClariTest
Centogene N.V. – CentoGenome, CentoXome, CentoArray, CentoMetabolic
Invitae Corp. – Invitae Rare Disorders Panel, Invitae Comprehensive Neuromuscular Disorders Panel
3billion, Inc. – Rare Disease Genetic Testing Panels, Whole Exome Sequencing (WES)
Arup Laboratories – ARUP Exome Test, Targeted NGS Panels for Rare Diseases
Eurofins Scientific – NMD Panel, Exome NGS for Rare Diseases
Strand Life Sciences – Strand RareGen, Whole Genome Sequencing for Rare Diseases
Ambry Genetics – Ambry Exome, Rare Disease NGS Panels
PerkinElmer, Inc. – PerkinElmer Genomics, Whole Genome Sequencing (WGS)
Realm IDX, Inc. – Rare Disease Exome Testing, Genomic Sequencing Panels
Macrogen, Inc. – Rare Disease NGS Panels, Whole Genome Sequencing
Baylor Genetics – Baylor Exome, Rare Disease Gene Panels
Color Genomics, Inc. – Color Genetic Risk Panels, Rare Disease Testing
Health Network Laboratories – HNL Genetic Testing Panels
Feb 2025: Strand Life Sciences, a subsidiary of Reliance Industries, launched the StrandOmics Portal, a digital platform aimed at enhancing rare disease diagnosis. The company also announced affordable genetic testing to improve accessibility and precision in rare disease detection.
Jan 2025: The Centre for Genetic Disorders at Banaras Hindu University (BHU) introduced new initiatives to enhance the research and diagnosis of genetic diseases in Varanasi. The development aims to improve access to advanced genetic testing and counseling.
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | USD 1.05 billion |
| Market Size by 2032 | USD 3.79 billion |
| CAGR | CAGR of 15.36% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Disease [Neurological Disorders, Immunological Disorders, Hematology Diseases, Endocrine & Metabolism Diseases, Cancer, Musculoskeletal Disorders, Cardiovascular Disorders, Dermatology Diseases, Other Rare Diseases] • By Technology [Next-generation sequencing, Array Technology, PCR-based Testing, FISH, Sanger Sequencing, Karyotyping] • By Specialty [Molecular Genetic Tests, Chromosomal Genetic Tests, Biochemical Genetic Tests] • By End Use [Research Laboratories & CROs, Hospitals & Clinics, Diagnostic Laboratories] |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | Quest Diagnostics Inc., Centogene N.V., Invitae Corp., 3billion Inc., Arup Laboratories, Eurofins Scientific, Strand Life Sciences, Ambry Genetics, PerkinElmer Inc., Realm IDX Inc., Macrogen Inc., Baylor Genetics, Color Genomics Inc., Health Network Laboratories. |
Ans: The Rare Disease Genetic Testing market is anticipated to grow at a CAGR of 15.36% from 2024 to 2032.
Ans: The market is expected to reach USD 1.05 billion by 2032, increasing from USD 3.79 billion in 2023.
Ans: The increasing prevalence of rare diseases is a major driver for the rare disease genetic testing market, with over 300 million people worldwide affected by these conditions.
Ans: The market faces significant restraints, primarily due to high costs and reimbursement limitations.
Ans: North America dominated the Rare Disease Genetic Testing market.
Table Of Contents
1. Introduction
1.1 Market Definition
1.2 Scope (Inclusion and Exclusions)
1.3 Research Assumptions
2. Executive Summary
2.1 Market Overview
2.2 Regional Synopsis
2.3 Competitive Summary
3. Research Methodology
3.1 Top-Down Approach
3.2 Bottom-up Approach
3.3. Data Validation
3.4 Primary Interviews
4. Market Dynamics Impact Analysis
4.1 Market Driving Factors Analysis
4.1.1 Drivers
4.1.2 Restraints
4.1.3 Opportunities
4.1.4 Challenges
4.2 PESTLE Analysis
4.3 Porter’s Five Forces Model
5. Statistical Insights and Trends Reporting
5.1 Incidence and Prevalence of Rare Diseases (2023)
5.2 Genetic Testing Utilization Trends (2023), by Region
5.3 Healthcare Spending on Rare Disease Genetic Testing, by Region (Government, Commercial, Private, Out-of-Pocket), 2023
5.4 Technological Advancements in Rare Disease Genetic Testing
5.5 Regulatory and Policy Trends Affecting Market Growth
5.6 Market Penetration and Accessibility of Genetic Testing
5.7 Patient and Physician Awareness Trends
6. Competitive Landscape
6.1 List of Major Companies, By Region
6.2 Market Share Analysis, By Region
6.3 Product Benchmarking
6.3.1 Product specifications and features
6.3.2 Pricing
6.4 Strategic Initiatives
6.4.1 Marketing and Promotional Activities
6.4.2 Distribution and Supply Chain Strategies
6.4.3 Expansion plans and new product launches
6.4.4 Strategic partnerships and collaborations
6.5 Technological Advancements
6.6 Market Positioning and Branding
7. Rare Disease Genetic Testing Market Segmentation, by Disease
7.1 Chapter Overview
7.2 Neurological Disorders
7.2.1 Neurological Disorders Market Trends Analysis (2020-2032)
7.2.2 Neurological Disorders Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3 Immunological Disorders
7.3.1 Immunological Disorders Market Trends Analysis (2020-2032)
7.3.2 Immunological Disorders Market Size Estimates and Forecasts to 2032 (USD Billion)
7.4 Hematology Diseases
7.4.1 Hematology Diseases Market Trends Analysis (2020-2032)
7.4.2 Hematology Diseases Market Size Estimates and Forecasts to 2032 (USD Billion)
7.5 Endocrine & Metabolism Diseases
7.5.1 Endocrine & Metabolism Diseases Market Trends Analysis (2020-2032)
7.5.2 Endocrine & Metabolism Diseases Market Size Estimates and Forecasts to 2032 (USD Billion)
7.6 Cancer
7.6.1 Cancer Market Trends Analysis (2020-2032)
7.6.2 Cancer Market Size Estimates and Forecasts to 2032 (USD Billion)
7.7 Musculoskeletal Disorders
7.7.1 Musculoskeletal Disorders Market Trends Analysis (2020-2032)
7.7.2 Musculoskeletal Disorders Market Size Estimates and Forecasts to 2032 (USD Billion)
7.8 Cardiovascular Disorders
7.8.1 Cardiovascular Disorders Market Trends Analysis (2020-2032)
7.8.2 Cardiovascular Disorders Market Size Estimates and Forecasts to 2032 (USD Billion)
7.9 Dermatology Diseases
7.9.1 Dermatology Diseases Market Trends Analysis (2020-2032)
7.9.2 Dermatology Diseases Market Size Estimates and Forecasts to 2032 (USD Billion)
7.10 Other Rare Diseases
7.10.1 Other Rare Diseases Market Trends Analysis (2020-2032)
7.10.2 Other Rare Diseases Market Size Estimates and Forecasts to 2032 (USD Billion)
8. Rare Disease Genetic Testing Market Segmentation, by Technology
8.1 Chapter Overview
8.2 Next-generation sequencing
8.2.1 Next-generation sequencing Market Trends Analysis (2020-2032)
8.2.2 Next-generation sequencing Market Size Estimates and Forecasts to 2032 (USD Billion)
8.3 Array Technology
8.3.1 Array Technology Market Trends Analysis (2020-2032)
8.3.2 Array Technology Market Size Estimates And Forecasts To 2032 (USD Billion)
8.4 PCR-based Testing
8.4.1 PCR-based Testing Market Trends Analysis (2020-2032)
8.4.2 PCR-based Testing Market Size Estimates And Forecasts To 2032 (USD Billion)
8.5 FISH
8.5.1 FISH Market Trends Analysis (2020-2032)
8.5.2 FISH Market Size Estimates And Forecasts To 2032 (USD Billion)
8.6 Sanger Sequencing
8.6.1 Sanger Sequencing Market Trends Analysis (2020-2032)
8.6.2 Sanger Sequencing Market Size Estimates And Forecasts To 2032 (USD Billion)
8.7 Karyotyping
8.7.1 Karyotyping Market Trends Analysis (2020-2032)
8.7.2 Karyotyping Market Size Estimates And Forecasts To 2032 (USD Billion)
9. Rare Disease Genetic Testing Market Segmentation, by Specialty
9.1 Chapter Overview
9.2 Molecular Genetic Tests
9.2.1 Molecular Genetic Tests Market Trends Analysis (2020-2032)
9.2.2 Molecular Genetic Tests Market Size Estimates and Forecasts to 2032 (USD Billion)
9.3 Chromosomal Genetic Tests
9.3.1 Chromosomal Genetic Tests Market Trends Analysis (2020-2032)
9.3.2 Chromosomal Genetic Tests Market Size Estimates and Forecasts to 2032 (USD Billion)
9.4 Biochemical Genetic Tests
9.4.1 Biochemical Genetic Tests Market Trends Analysis (2020-2032)
9.4.2 Biochemical Genetic Tests Market Size Estimates and Forecasts to 2032 (USD Billion)
10. Rare Disease Genetic Testing Market Segmentation, By End-Use
10.1 Chapter Overview
10.2 Research Laboratories & CROs
10.2.1 Research Laboratories & CROs Market Trends Analysis (2020-2032)
10.2.2 Research Laboratories & CROs Market Size Estimates and Forecasts to 2032 (USD Billion)
10.3 Hospitals & Clinics
10.3.1 Hospitals & Clinics Market Trends Analysis (2020-2032)
10.3.2 Hospitals & Clinics Market Size Estimates and Forecasts to 2032 (USD Billion)
10.4 Diagnostic Laboratories
10.4.1 Diagnostic Laboratories Market Trends Analysis (2020-2032)
10.4.2 Diagnostic Laboratories Market Size Estimates and Forecasts to 2032 (USD Billion)
11. Regional Analysis
11.1 Chapter Overview
11.2 North America
11.2.1 Trends Analysis
11.2.2 North America Rare Disease Genetic Testing Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
11.2.3 North America Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.2.4 North America Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.2.5 North America Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.2.6 North America Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.2.7 USA
11.2.7.1 USA Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.2.7.2 USA Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.2.7.3 USA Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.2.7.4 USA Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.2.8 Canada
11.2.8.1 Canada Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.2.8.2 Canada Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.2.8.3 Canada Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.2.8.4 Canada Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.2.9 Mexico
11.2.9.1 Mexico Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.2.9.2 Mexico Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.2.9.3 Mexico Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.2.9.4 Mexico Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion
11.3 Europe
11.3.1 Eastern Europe
11.3.1.1 Trends Analysis
11.3.1.2 Eastern Europe Rare Disease Genetic Testing Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
11.3.1.3 Eastern Europe Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.3.1.4 Eastern Europe Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.3.1.5 Eastern Europe Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.3.1.6 Eastern Europe Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.3.1.7 Poland
11.3.1.7.1 Poland Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.3.1.7.2 Poland Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.3.1.7.3 Poland Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.3.1.7.4 Poland Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.3.1.8 Romania
11.3.1.8.1 Romania Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.3.1.8.2 Romania Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.3.1.8.3 Romania Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.3.1.8.4 Romania Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.3.1.9 Hungary
11.3.1.9.1 Hungary Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.3.1.9.2 Hungary Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.3.1.9.3 Hungary Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.3.1.9.4 Hungary Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.3.1.10 Turkey
11.3.1.10.1 Turkey Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.3.1.10.2 Turkey Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.3.1.10.3 Turkey Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.3.1.10.4 Turkey Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.3.1.11 Rest of Eastern Europe
11.3.1.11.1 Rest of Eastern Europe Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.3.1.11.2 Rest of Eastern Europe Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.3.1.11.3 Rest of Eastern Europe Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.3.1.11.4 Rest of Eastern Europe Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.3.2 Western Europe
11.3.2.1 Trends Analysis
11.3.2.2 Western Europe Rare Disease Genetic Testing Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
11.3.2.3 Western Europe Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.3.2.4 Western Europe Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.3.2.5 Western Europe Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.3.2.6 Western Europe Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.3.2.7 Germany
11.3.2.7.1 Germany Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.3.2.7.2 Germany Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.3.2.7.3 Germany Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.3.2.7.4 Germany Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.3.2.8 France
11.3.2.8.1 France Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.3.2.8.2 France Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.3.2.8.3 France Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.3.2.8.4 France Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.3.2.9 UK
11.3.2.9.1 UK Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.3.2.9.2 UK Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.3.2.9.3 UK Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.3.2.9.4 UK Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.3.2.10 Italy
11.3.2.10.1 Italy Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.3.2.10.2 Italy Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.3.2.10.3 Italy Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.3.2.10.4 Italy Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.3.2.11 Spain
11.3.2.11.1 Spain Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.3.2.11.2 Spain Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.3.2.11.3 Spain Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.3.2.11.4 Spain Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.3.2.12 Netherlands
11.3.2.12.1 Netherlands Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.3.2.12.2 Netherlands Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.3.2.12.3 Netherlands Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.3.2.12.4 Netherlands Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.3.2.13 Switzerland
11.3.2.13.1 Switzerland Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.3.2.13.2 Switzerland Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.3.2.13.3 Switzerland Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.3.2.13.4 Switzerland Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.3.2.14 Austria
11.3.2.14.1 Austria Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.3.2.14.2 Austria Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.3.2.14.3 Austria Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.3.2.14.4 Austria Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.3.2.15 Rest of Western Europe
11.3.2.15.1 Rest of Western Europe Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.3.2.15.2 Rest of Western Europe Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.3.2.15.3 Rest of Western Europe Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.3.2.15.4 Rest of Western Europe Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.4 Asia Pacific
11.4.1 Trends Analysis
11.4.2 Asia Pacific Rare Disease Genetic Testing Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
11.4.3 Asia Pacific Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.4.4 Asia Pacific Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.4.5 Asia Pacific Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.4.6 Asia Pacific Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.4.7 China
11.4.7.1 China Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.4.7.2 China Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.4.7.3 China Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.4.7.4 China Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.4.8 India
11.4.8.1 India Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.4.8.2 India Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.4.8.3 India Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.4.8.4 India Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.4.9 Japan
11.4.9.1 Japan Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.4.9.2 Japan Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.4.9.3 Japan Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.4.9.4 Japan Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.4.10 South Korea
11.4.10.1 South Korea Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.4.10.2 South Korea Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.4.10.3 South Korea Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.4.10.4 South Korea Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.4.11 Vietnam
11.4.11.1 Vietnam Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.4.11.2 Vietnam Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.4.11.3 Vietnam Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.4.11.4 Vietnam Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.4.12 Singapore
11.4.12.1 Singapore Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.4.12.2 Singapore Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.4.12.3 Singapore Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.4.12.4 Singapore Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.4.13 Australia
11.4.13.1 Australia Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.4.13.2 Australia Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.4.13.3 Australia Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.4.13.4 Australia Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.4.14 Rest of Asia Pacific
11.4.14.1 Rest of Asia Pacific Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.4.14.2 Rest of Asia Pacific Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.4.14.3 Rest of Asia Pacific Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.4.14.4 Rest of Asia Pacific Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.5 Middle East and Africa
11.5.1 Middle East
11.5.1.1 Trends Analysis
11.5.1.2 Middle East Rare Disease Genetic Testing Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
11.5.1.3 Middle East Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.5.1.4 Middle East Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.5.1.5 Middle East Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.5.1.6 Middle East Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.5.1.7 UAE
11.5.1.7.1 UAE Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.5.1.7.2 UAE Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.5.1.7.3 UAE Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.5.1.7.4 UAE Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.5.1.8 Egypt
11.5.1.8.1 Egypt Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.5.1.8.2 Egypt Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.5.1.8.3 Egypt Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.5.1.8.4 Egypt Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.5.1.9 Saudi Arabia
11.5.1.9.1 Saudi Arabia Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.5.1.9.2 Saudi Arabia Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.5.1.9.3 Saudi Arabia Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.5.1.9.4 Saudi Arabia Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.5.1.10 Qatar
11.5.1.10.1 Qatar Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.5.1.10.2 Qatar Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.5.1.10.3 Qatar Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.5.1.10.4 Qatar Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.5.1.11 Rest of Middle East
11.5.1.11.1 Rest of Middle East Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.5.1.11.2 Rest of Middle East Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.5.1.11.3 Rest of Middle East Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.5.1.11.4 Rest of Middle East Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.5.2 Africa
11.5.2.1 Trends Analysis
11.5.2.2 Africa Rare Disease Genetic Testing Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
11.5.2.3 Africa Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.5.2.4 Africa Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.5.2.5 Africa Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.5.2.6 Africa Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.5.2.7 South Africa
11.5.2.7.1 South Africa Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.5.2.7.2 South Africa Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.5.2.7.3 South Africa Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.5.2.7.4 South Africa Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.5.2.8 Nigeria
11.5.2.8.1 Nigeria Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.5.2.8.2 Nigeria Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.5.2.8.3 Nigeria Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.5.2.8.4 Nigeria Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.5.2.9 Rest of Africa
11.5.2.9.1 Rest of Africa Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.5.2.9.2 Rest of Africa Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.5.2.9.3 Rest of Africa Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.5.2.9.4 Rest of Africa Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.6 Latin America
11.6.1 Trends Analysis
11.6.2 Latin America Rare Disease Genetic Testing Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
11.6.3 Latin America Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.6.4 Latin America Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.6.5 Latin America Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.6.6 Latin America Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.6.7 Brazil
11.6.7.1 Brazil Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.6.7.2 Brazil Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.6.7.3 Brazil Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.6.7.4 Brazil Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.6.8 Argentina
11.6.8.1 Argentina Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.6.8.2 Argentina Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.6.8.3 Argentina Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.6.8.4 Argentina Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.6.9 Colombia
11.6.9.1 Colombia Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.6.9.2 Colombia Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.6.9.3 Colombia Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.6.9.4 Colombia Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
11.6.10 Rest of Latin America
11.6.10.1 Rest of Latin America Rare Disease Genetic Testing Market Estimates and Forecasts, by Disease (2020-2032) (USD Billion)
11.6.10.2 Rest of Latin America Rare Disease Genetic Testing Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11.6.10.3 Rest of Latin America Rare Disease Genetic Testing Market Estimates and Forecasts, by Specialty (2020-2032) (USD Billion)
11.6.10.4 Rest of Latin America Rare Disease Genetic Testing Market Estimates and Forecasts, By End-Use (2020-2032) (USD Billion)
12. Company Profiles
12.1 Quest Diagnostics Inc.
12.1.1 Company Overview
12.1.2 Financial
12.1.3 Product / Services Offered
12.1.4 SWOT Analysis
12.2 Centogene N.V.
12.2.1 Company Overview
12.2.2 Financial
12.2.3 Product / Services Offered
12.2.4 SWOT Analysis
12.3 Invitae Corp.
12.3.1 Company Overview
12.3.2 Financial
12.3.3 Product / Services Offered
12.3.4 SWOT Analysis
12.4 3billion, Inc.
12.4.1 Company Overview
12.4.2 Financial
12.4.3 Product / Services Offered
12.4.4 SWOT Analysis
12.5 Arup Laboratories
12.5.1 Company Overview
12.5.2 Financial
12.5.3 Product / Services Offered
12.5.4 SWOT Analysis
12.6 Eurofins Scientific
12.6.1 Company Overview
12.6.2 Financial
12.6.3 Product / Services Offered
12.6.4 SWOT Analysis
12.7 Strand Life Sciences
12.7.1 Company Overview
12.7.2 Financial
12.7.3 Product / Services Offered
12.7.4 SWOT Analysis
12.8 Ambry Genetics
12.8.1 Company Overview
12.8.2 Financial
12.8.3 Product / Services Offered
12.8.4 SWOT Analysis
12.9 PerkinElmer, Inc.
12.9.1 Company Overview
12.9.2 Financial
12.9.3 Product / Services Offered
12.9.4 SWOT Analysis
12.10 Realm IDX, Inc.
12.10.1 Company Overview
12.10.2 Financial
12.10.3 Product / Services Offered
12.10.4 SWOT Analysis
13. Use Cases and Best Practices
14. Conclusion
An accurate research report requires proper strategizing as well as implementation. There are multiple factors involved in the completion of good and accurate research report and selecting the best methodology to compete the research is the toughest part. Since the research reports we provide play a crucial role in any company’s decision-making process, therefore we at SNS Insider always believe that we should choose the best method which gives us results closer to reality. This allows us to reach at a stage wherein we can provide our clients best and accurate investment to output ratio.
Each report that we prepare takes a timeframe of 350-400 business hours for production. Starting from the selection of titles through a couple of in-depth brain storming session to the final QC process before uploading our titles on our website we dedicate around 350 working hours. The titles are selected based on their current market cap and the foreseen CAGR and growth.
The 5 steps process:
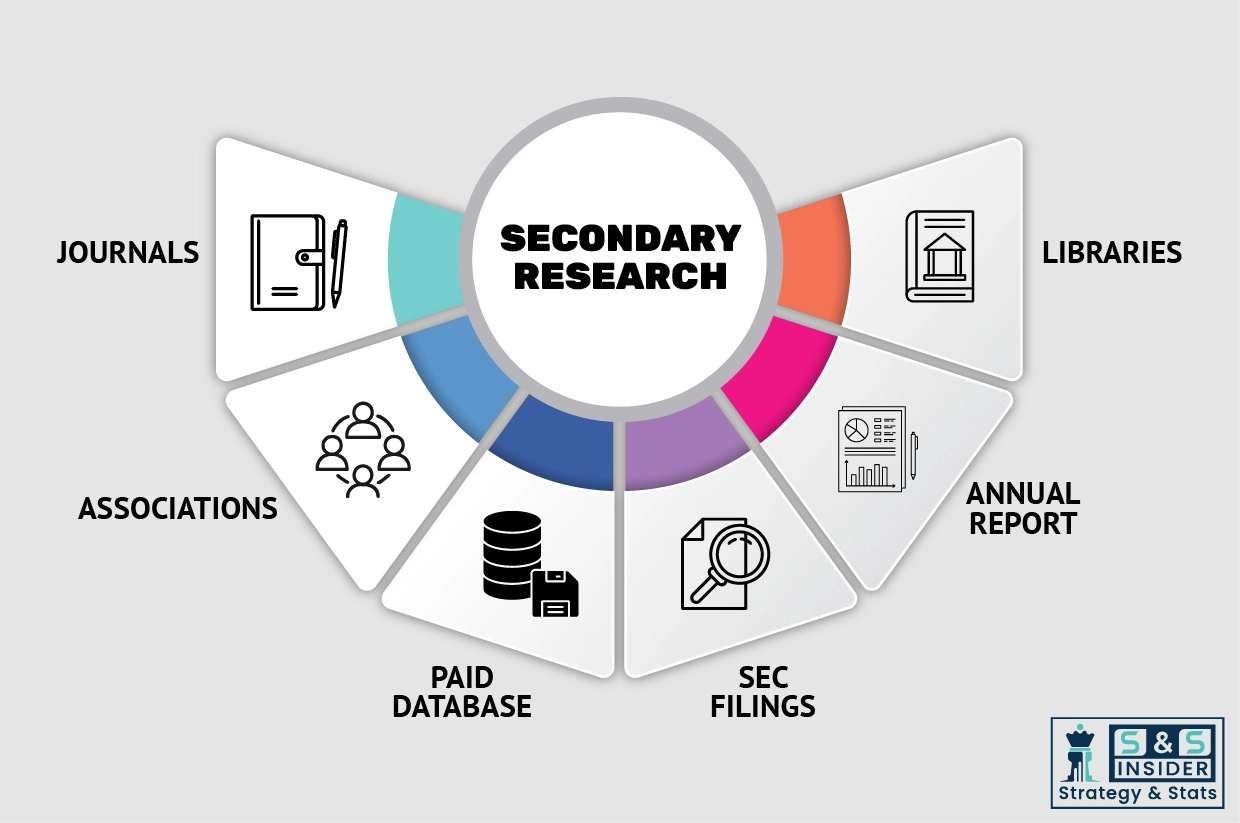
Step 1: Secondary Research:
Secondary Research or Desk Research is as the name suggests is a research process wherein, we collect data through the readily available information. In this process we use various paid and unpaid databases which our team has access to and gather data through the same. This includes examining of listed companies’ annual reports, Journals, SEC filling etc. Apart from this our team has access to various associations across the globe across different industries. Lastly, we have exchange relationships with various university as well as individual libraries.
Step 2: Primary Research
When we talk about primary research, it is a type of study in which the researchers collect relevant data samples directly, rather than relying on previously collected data. This type of research is focused on gaining content specific facts that can be sued to solve specific problems. Since the collected data is fresh and first hand therefore it makes the study more accurate and genuine.
We at SNS Insider have divided Primary Research into 2 parts.
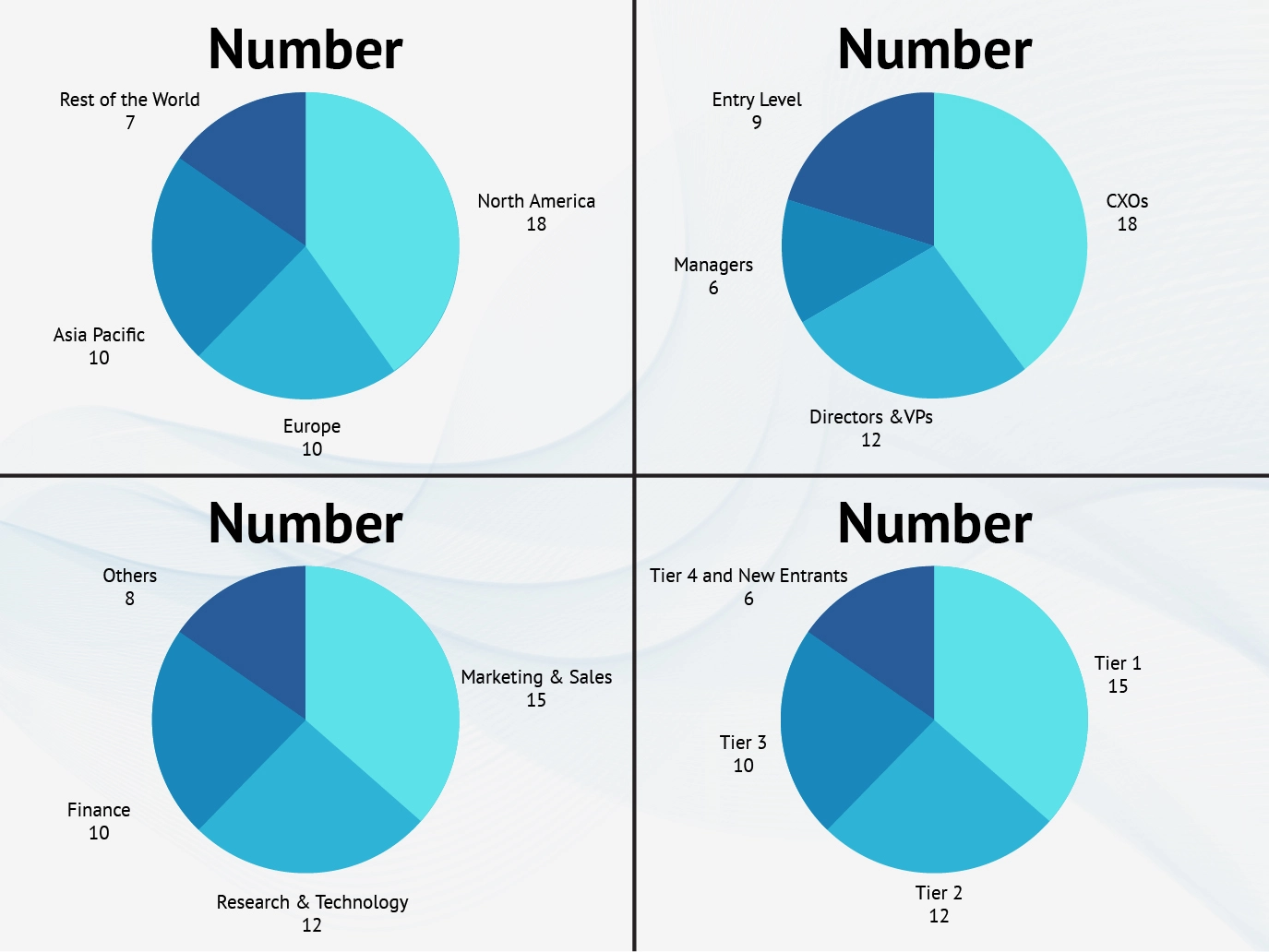
Part 1 wherein we interview the KOLs of major players as well as the upcoming ones across various geographic regions. This allows us to have their view over the market scenario and acts as an important tool to come closer to the accurate market numbers. As many as 45 paid and unpaid primary interviews are taken from both the demand and supply side of the industry to make sure we land at an accurate judgement and analysis of the market.
This step involves the triangulation of data wherein our team analyses the interview transcripts, online survey responses and observation of on filed participants. The below mentioned chart should give a better understanding of the part 1 of the primary interview.
Part 2: In this part of primary research the data collected via secondary research and the part 1 of the primary research is validated with the interviews from individual consultants and subject matter experts.
Consultants are those set of people who have at least 12 years of experience and expertise within the industry whereas Subject Matter Experts are those with at least 15 years of experience behind their back within the same space. The data with the help of two main processes i.e., FGDs (Focused Group Discussions) and IDs (Individual Discussions). This gives us a 3rd party nonbiased primary view of the market scenario making it a more dependable one while collation of the data pointers.
Step 3: Data Bank Validation
Once all the information is collected via primary and secondary sources, we run that information for data validation. At our intelligence centre our research heads track a lot of information related to the market which includes the quarterly reports, the daily stock prices, and other relevant information. Our data bank server gets updated every fortnight and that is how the information which we collected using our primary and secondary information is revalidated in real time.

Step 4: QA/QC Process
After all the data collection and validation our team does a final level of quality check and quality assurance to get rid of any unwanted or undesired mistakes. This might include but not limited to getting rid of the any typos, duplication of numbers or missing of any important information. The people involved in this process include technical content writers, research heads and graphics people. Once this process is completed the title gets uploader on our platform for our clients to read it.
Step 5: Final QC/QA Process:
This is the last process and comes when the client has ordered the study. In this process a final QA/QC is done before the study is emailed to the client. Since we believe in giving our clients a good experience of our research studies, therefore, to make sure that we do not lack at our end in any way humanly possible we do a final round of quality check and then dispatch the study to the client.
By Disease
Neurological Disorders
Immunological Disorders
Hematology Diseases
Endocrine & Metabolism Diseases
Cancer
Musculoskeletal Disorders
Cardiovascular Disorders
Dermatology Diseases
Other Rare Diseases
By Technology
Next-generation sequencing
Array Technology
PCR-based Testing
FISH
Sanger Sequencing
Karyotyping
By Specialty
Molecular Genetic Tests
Chromosomal Genetic Tests
Biochemical Genetic Tests
By End Use
Research Laboratories & CROs
Hospitals & Clinics
Diagnostic Laboratories
Request for Segment Customization as per your Business Requirement: Segment Customization Request
Regional Coverage:
North America
US
Canada
Mexico
Europe
Eastern Europe
Poland
Romania
Hungary
Turkey
Rest of Eastern Europe
Western Europe
Germany
France
UK
Italy
Spain
Netherlands
Switzerland
Austria
Rest of Western Europe
Asia Pacific
China
India
Japan
South Korea
Vietnam
Singapore
Australia
Rest of Asia Pacific
Middle East & Africa
Middle East
UAE
Egypt
Saudi Arabia
Qatar
Rest of Middle East
Africa
Nigeria
South Africa
Rest of Africa
Latin America
Brazil
Argentina
Colombia
Rest of Latin America
Request for Country Level Research Report: Country Level Customization Request
Available Customization
With the given market data, SNS Insider offers customization as per the company’s specific needs. The following customization options are available for the report:
Detailed Volume Analysis
Criss-Cross segment analysis (e.g. Product X Application)
Competitive Product Benchmarking
Geographic Analysis
Additional countries in any of the regions
Customized Data Representation
Detailed analysis and profiling of additional market players