Rapid Tests Market Size & Report Overview:
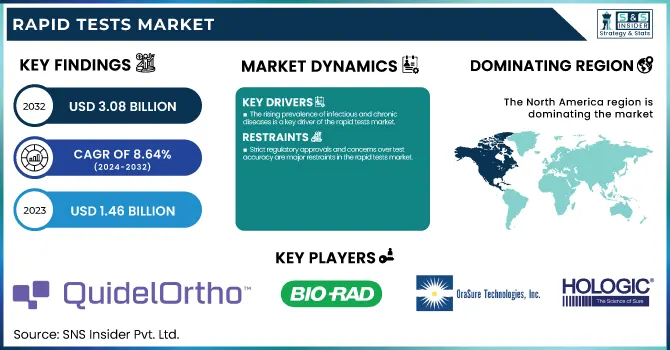
The Rapid Tests Market was valued at USD 1.46 billion in 2023 and is expected to reach USD 3.08 billion by 2032, with a CAGR of 8.64% from 2024 to 2032.
To Get more information on Rapid Tests Market - Request Free Sample Report
This study emphasizes the growing usage of rapid tests in various regions due to increasing healthcare awareness, demand for speed in diagnostics, and widening applications in infectious disease screening and chronic disease management. Price trends and cost analysis are discussed in this study as it investigates affordability, reimbursement policies, and manufacturing processes that affect product availability. Furthermore, it explores innovation and R&D directions in rapid testing, particularly in creating AI-embedded diagnostic equipment, enhanced accuracy, and future point-of-care technologies that promote convenience and efficiency in healthcare.
Rapid Tests Market Dynamics
Drivers
-
The rising prevalence of infectious and chronic diseases is a key driver of the rapid tests market.
Infectious diseases like influenza, HIV, tuberculosis, and COVID-19 need to be diagnosed rapidly and accurately to contain outbreaks and receive timely treatments. For instance, according to WHO, more than 10.6 million cases of tuberculosis were reported in 2021, highlighting the necessity for rapid TB diagnostic testing solutions. Moreover, non-communicable diseases like diabetes and cardiovascular diseases have boosted the demand for home and point-of-care (POC) testing solutions. The increasing popularity of pregnancy, glucose, and infectious disease self-test kits further boosts the market. Advances in lateral flow assays, molecular diagnostics, and rapid tests with AI integration have enhanced accuracy and ease of use, making them more affordable for healthcare professionals as well as consumers. Governments across the globe are also encouraging rapid diagnostic testing by way of subsidies, funding schemes, and approvals for home-based kits. The transition to decentralized models of healthcare and telemedicine has also boosted demand further. Additionally, increased knowledge among consumers and healthcare professionals regarding the advantages of early disease identification has driven stronger adoption of rapid tests, particularly in hospitals, clinics, and homes.
Restraints
-
Strict regulatory approvals and concerns over test accuracy are major restraints in the rapid tests market.
Government regulatory authorities like the U.S. FDA, European Medicines Agency (EMA), and WHO have strict validation guidelines for diagnostic tests, resulting in slow approval times. This slows down new product introduction to the market and raises the cost of development. Variability in regulatory needs across nations also results in compliance issues, discouraging international expansion by companies. A further limitation is test accuracy issues, especially with antigen-based and home-use tests. For instance, antigen tests for COVID-19 were less sensitive than PCR tests and produced false-negative results in a few instances. These concerns regarding accuracy affect the legitimacy and use of rapid tests in high-stakes healthcare use cases. Again, the high price of high-end molecular diagnostic tests makes them inaccessible, particularly in poor communities and small hospitals. Rapid test reimbursement policies are also inconsistent, with healthcare systems in some countries reimbursing laboratory-based diagnostics but placing caps on the reimbursement of home-based testing kits. In addition, variability in quality across various brands and companies complicates ensuring the reliability of tests. Such issues cumulatively hinder rapid test adoption across some segments despite the expanding market prospects.
Opportunities
-
The rising popularity of at-home diagnostic testing and technological innovations present significant growth opportunities in the rapid tests market.
The popularity of COVID-19 self-testing kits has sped up consumer adoption of home diagnostics, and the demand for rapid tests for conditions like diabetes monitoring, pregnancy testing, sexually transmitted diseases (STIs), and respiratory infections is increasing. Companies are increasingly making investments in intelligent diagnostic equipment that is compatible with mobile applications and cloud platforms for real-time remote patient monitoring. Advances in AI-driven diagnostic algorithms, CRISPR tests, and biosensors are making rapid tests more accurate and efficient, pushing the scope of these tests from infectious diseases to oncology and genetic disorders. Further, government-backed programs for early disease detection programs and decentralized healthcare services are also fueling market growth. For instance, numerous nations are investing in national screening initiatives for conditions like HIV, hepatitis, and antimicrobial resistance (AMR), boosting the demand for portable and rapid testing solutions. Combining telemedicine with home-based testing kits is also further increasing access, allowing patients to remotely consult healthcare professionals while using rapid test results for diagnosis. With increasing digital health adoption, the convergence of speedy diagnostics and connected health solutions will generate new streams of revenue for industry participants.
Challenges
-
The rapid tests market faces challenges such as intense pricing competition, manufacturing constraints, and supply chain vulnerabilities.
With some global and regional competitors providing comparable rapid diagnostic solutions, companies are subjected to pricing pressure that compromises profit margins. Developing economies have cost-sensitive markets that opt for cheap rapid tests, and companies find it hard to remain profitable while maintaining quality. The fact that raw materials like antibodies, reagents, and molecular diagnostic components are costly also affects the cost of production. Supply chain disruptions, especially in times of international health emergencies such as the COVID-19 pandemic, have raised concerns about the procurement of critical materials. Diagnostic firms often depend on third-party vendors to provide test components, exposing them to more potential logistical shortages and delays. Consumer distrust in the reliability of test results—while accuracy has improved, some consumers still prefer laboratory-based testing because of false positives or negative concerns—poses another challenge. Additionally, limited awareness in rural and underdeveloped regions hinders market growth since most communities are still dependent on traditional diagnostics. To counter these issues, firms need to concentrate on low-cost manufacturing, supply chain agility, and consumer education programs to gain the trust of rapid testing solutions.
Rapid Tests Market Segmentation Analysis
By Product
The Consumables segment was the market leader in the rapid tests market during 2023, accounting for 69.08% of the overall revenue. This leadership is primarily fuelled by the strong demand for single-use test kits, reagents, and cartridges required for conducting rapid diagnostics. Consumables are a major component of point-of-care testing, home-based self-testing, and professional medical diagnostics, which makes them critical to the sector. The outbreak of infectious diseases like COVID-19, influenza, and sexually transmitted diseases has increased the demand for consumables, especially in decentralized healthcare environments. The growing application of lateral flow assays, immunoassay kits, and molecular diagnostic test strips has also driven segment growth. With healthcare providers and consumers both seeking quicker, more convenient diagnostic solutions, the use of consumables continues to increase. The growth in self-testing trends, fueled by increased public health consciousness and availability through pharmacies and online websites, further cements the segment's leadership.
The most rapidly growing segment is Instruments, as advancements in automated diagnostic platforms are picking up steam. Instruments like portable analyzers and handheld instruments are gaining popularity, especially in hospital and laboratory environments where precision and efficiency are more important. The increasing use of digital and AI-based rapid testing platforms is likely to drive this segment's growth.
By Technology
The Immunoassay segment dominated the rapid tests market in 2023 with a share of 53.45% of total revenue. Immunoassays are still the most common technology employed in rapid testing because they are affordable, easy to use, and have fast turnaround times. Immunoassays are widely employed for the diagnosis of infectious diseases, cardiac ailments, pregnancy, and drug abuse and are therefore an integral component of both clinical and home diagnostic solutions. Imunoassay-based tests, like lateral flow and enzyme-linked immunosorbent assays (ELISA), are widely used based on their robustness and scale-up. The COVID-19 pandemic helped promote the need for immunoassay-based quick tests at an accelerated pace, especially for detecting antigens and antibodies. The growing number of chronic diseases and infectious disorders have also contributed to the use of immunoassay-based rapid tests in hospital settings, clinical laboratories, and home environments.
The most rapidly expanding part is Molecular Diagnostics, which is gathering pace because of higher accuracy and sensitivity. Molecular diagnostics, especially polymerase chain reaction (PCR) and isothermal amplification methods, are being more highly sought after for the detection of respiratory infections, antibiotic resistance, and new viral diseases. Increasing demand for accurate, real-time diagnostic outcomes and continued innovations in portable molecular testing equipment are propelling fast uptake of this technology.
By Application
The Upper Respiratory Tract Infections segment accounted for the majority of the rapid tests market in 2023, capturing 59.7% of the total revenue. The rising prevalence of respiratory infections, including influenza, COVID-19, respiratory syncytial virus (RSV), and common colds, has heavily driven the demand for rapid tests. During the pandemic, home and point-of-care rapid antigen and molecular tests were made readily available, thus resulting in increased adoption. Also, hospitals, clinics, and pharmacies have grown to depend more on quick tests to promptly diagnose respiratory infections and apply on-time treatments, lessening hospitalization and transmission rates. Seasonal outbreaks of flu and increased air pollution rates, causing respiratory illnesses, have also fueled market growth. The ease, economy, and high availability of fast respiratory infection testing via retail stores and the Internet have made such tests a chosen diagnostic tool both for healthcare workers and consumers alike.
The most rapidly expanding segment is Antibiotic-Resistant Infections, as antimicrobial resistance (AMR) emerges as a rapidly increasing global threat. The requirement for quick and precise identification of resistant bacterial strains is fueling demand for rapid specialized tests. Governments and healthcare institutions across the globe are compelling the development of improved AMR detection technologies, and this segment is one of the fastest-growing in the rapid diagnostics market.
By End-Use
The Hospitals & Clinics segment was the leader in the rapid tests market in 2023 with a 42.9% share in revenue. The reason behind the segment's leadership is the immense patient volume in hospital and clinical environments, where rapid diagnostic tests are regularly applied for rapid detection of disease and planning treatment. Advanced medical infrastructure, professional healthcare providers, and regulatory requirements are available in hospitals to guarantee correct and prompt diagnosis, thereby cementing the leadership of the segment. Hospital and clinic rapid tests are utilized mostly for acute diagnosis, infectious disease screening, pregnancy diagnosis, and chronic disease management. The rising epidemiologic burden of infectious diseases combined with the desire for rapid turnaround has motivated hospital and clinic laboratories to embrace rapid testing extensively. Also, reimbursement policy and healthcare government initiatives have facilitated the extension of rapid testing to institutional care settings.
The most rapidly expanding segment is At-Home Testing, which is witnessing a huge upsurge in demand due to increasing consumer preference for self-testing. The ease, low cost, and privacy provided by at-home rapid test kits for diseases like COVID-19, flu, pregnancy, and sexually transmitted diseases (STIs) have driven the market growth. Moreover, advancements in digital health integration, such as smartphone-enabled diagnostic kits, are also pushing this segment's growth at a rapid pace.
Rapid Tests Market Regional Insights
North America accounted for the largest market share, led mainly by the high rate of point-of-care diagnostic adoption, good distribution of leading market players, and facilitation by regulatory frameworks. The U.S. dominates the region due to the growing need for rapid tests in infectious disease detection, chronic disease management, and home-based self-testing. Government programs to enhance diagnostic access and continued technological innovation also support growth in the market.
Europe came next as a significant market, with Germany, the U.K., and France at the forefront of adopting quick tests. Europe is favored by robust healthcare policies, wide research on molecular diagnostics, and increased demand for antimicrobial resistance (AMR) testing. Telehealth services and home diagnostics expansion are also driving the market forward.
The Asia-Pacific region is growing fastest because of rising investments in healthcare, growing awareness of rapid diagnostics, and the escalating burden of infectious diseases such as tuberculosis, dengue, and COVID-19. There is a surge in demand in countries such as China, India, and Japan, especially for self-testing kits and point-of-care diagnostics. Government initiatives aiding disease surveillance and early detection also contribute to growth.
Get Customized Report as per Your Business Requirement - Enquiry Now
Key Players in the Rapid Tests Market
-
QuidelOrtho Corporation – Sofia, QuickVue, AmpliVue
-
OraSure Technologies, Inc. – OraQuick
-
Bio-Rad Laboratories, Inc. – BioPlex 2200, D-10 Hemoglobin Testing System
-
Hologic, Inc. – Aptima, Panther Fusion
-
Meridian Bioscience – Illumigene, Meridian
-
Akers Biosciences, Inc. – BreathScan, PIFA Heparin/PF4
-
Romer Labs – AgraStrip, RapidChek
-
Eurofins – EmpowerDX, Transplant Genomics
-
Intertek – Intertek COVID-19 PCR Testing
-
Bureau Veritas – BV COVID-19 Testing Solutions
-
TUV SUD – TÜV SÜD Testing Services
-
ALS Limited – ALS Food and Pharmaceutical Testing
-
Microbac Laboratories – Microbac COVID-19 Testing
-
AsureQuality – AsureQuality Food Testing
-
Genetic ID – Genetic ID DNA Testing
-
OMIC USA – OMIC Allergen Testing
Recent Developments
In Dec 2024, OraSure Technologies, Inc. secured federal funding through the Rapid Response Partnership Vehicle (RRPV) to develop a rapid antigen test for Marburg Virus Disease (MVD).
In Dec 2024, Hologic, Inc. agreed with the CDC to develop analyte-specific reagents (ASRs) for detecting H5N1 bird flu. These ASRs will serve as key components in laboratory-developed tests, enhancing disease identification and surveillance.
In April 2024, QuidelOrtho Corporation received FDA 510(k) clearance for its QuickVue COVID-19 test, enabling its use in homes and medical facilities with CLIA waivers. This milestone underscores the company's commitment to advancing diagnostic solutions and strengthening market competitiveness.
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | USD 1.46 billion |
| Market Size by 2032 | USD 3.08 billion |
| CAGR | CAGR of 8.64% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Product [Instruments, Consumables, Others] • By Technology [Immunoassay, Molecular diagnostics, Other technologies] • By Application [Upper respiratory tract infections (Influenza and Parainfluenza virus, Streptococcus, Respiratory syncytial virus), Antibiotic-resistant infections, Sepsis (Bacterial Sepsis, Fungal Sepsis, Others)] • By End-Use [Hospitals & clinics, Laboratories, At-home testing and others] |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | QuidelOrtho Corporation, OraSure Technologies, Inc., Bio-Rad Laboratories, Inc., Hologic, Inc., Meridian Bioscience, Akers Biosciences, Inc., Romer Labs, Eurofins, Intertek, Bureau Veritas, TUV SUD, ALS Limited, Microbac Laboratories, AsureQuality, Genetic ID, OMIC USA. |

