Prostate Cancer Diagnostics Market Size & Trends:
The Prostate Cancer Diagnostics Market was valued at USD 8.57 billion in 2023 and is projected to reach USD 15.09 billion by 2032, growing at a CAGR of 6.50% from 2024-2032.
To Get more information on Prostate Cancer Diagnostics Market - Request Free Sample Report
The Prostate Cancer Diagnostics Market report provides unique statistical data beyond typical growth analysis. It gives comprehensive incidence and prevalence data (2023) for major regions, which helps in a better understanding of disease burden. We provide regional diagnostic test adoption trends, including the transition to sophisticated biomarker-based testing. The report further provides diagnostic test volume analysis, projecting demand fluctuations. Also, our research examines healthcare expenditure distribution by government, commercial, private, and out-of-pocket segments, providing a complete financial picture of prostate cancer diagnosis. These distinguishing features render our report an evidence-based tool for strategic decision-making.
Prostate Cancer Diagnostics Market Dynamics
Drivers
-
Rising Prevalence of Prostate Cancer and Growing Geriatric Population Accelerating the Prostate Cancer Diagnostics Market.
The growing incidence of prostate cancer worldwide is a major growth driver for the prostate cancer diagnostics market. Prostate cancer is the second most prevalent cancer in men across the globe, with more than 1.4 million new cases reported in 2023, as per the World Cancer Research Fund. The incidence of the disease is notably high in the developed world of North America and Europe, where widespread screening programs and improved healthcare facilities allow for early diagnosis. Moreover, the expanding geriatric population is fueling market growth, given that prostate cancer mainly happens in men aged above 50. According to the United Nations, by the year 2050, one in every six persons worldwide will be aged 65 and above, boosting demand for diagnostic products. Progress in PSA testing, genetic biomarkers, and imaging modalities like multiparametric MRI is also driving the market forward.
-
Advancements in Liquid Biopsy and Genomic Testing propelling the market growth.
The increasing use of liquid biopsy and genomic testing for the diagnosis of prostate cancer is revolutionizing the market dynamics. Liquid biopsy, which allows for non-invasive identification of circulating tumor DNA (ctDNA) and RNA biomarkers, is picking up pace as a more patient-friendly and accessible option compared to conventional biopsies. A recent 2023 Nature Medicine study emphasized that liquid biopsy tests were able to identify prostate cancer with an 85% accuracy rate, enhancing early detection. Moreover, advancements in genomic testing, including Next-Generation Sequencing (NGS) and AI-based biomarker discovery, are facilitating more accurate and individualized diagnostics. Firms such as Exact Sciences, Myriad Genetics, and MDxHealth are actively introducing novel prostate cancer genomic tests, refining risk stratification, and informing targeted treatment decisions. These advances improve diagnostic efficiency, propelling increased adoption in the clinic.
Restraint
-
High Cost and Limited Accessibility of Advanced Diagnostic Tests restraining the market growth.
The expense and rarity of sophisticated prostate cancer diagnostic tests are a major hindrance to market growth, especially in emerging markets. Sophisticated diagnostic techniques like multiparametric MRI, liquid biopsy, and genomic tests are highly accurate but usually pricey, restricting their mass usage. For example, a Prolaris genomic test could be between USD 3,400 and USD 4,000, which is out of reach for most patients without comprehensive insurance. Also, low- and middle-income nations lack adequate healthcare infrastructure and inadequate reimbursement policies to limit access to advanced diagnostic solutions. Government programs and investment by the private sector are intended to fill this gap, but the gaps in healthcare access continue to be a challenge. The requirement for cost-effective and affordable screening solutions remains a top concern, hindering the overall market penetration of innovative diagnostics.
Opportunities
-
The convergence of artificial intelligence (AI) and machine learning (ML) algorithms in prostate cancer diagnosis is a significant market growth opportunity.
AI-based diagnostic devices can interpret medical images, pathology slides, and genomic information with high accuracy, minimizing diagnostic mistakes and enhancing early cancer detection. Firms such as IBM Watson Health and Paige AI are placing bets on AI-based pathology platforms that improve multiparametric MRI (mpMRI) and biopsy analysis accuracy. Current research reveals that AI-assisted imaging could raise prostate cancer detection rates by 20-30% relative to conventional means. Additionally, AI can also differentiate between aggressive and indolent tumors and reduce overdiagnosis and over-treatment. With the increasing adoption of AI-powered diagnostic platforms, they can revolutionize the detection of prostate cancer by making diagnosis quicker, more precise, and affordable.
Challenges
-
Variability in Diagnostic Accuracy and Overdiagnosis Risks challenging the market.
One of the biggest issues in the Prostate Cancer Diagnostics Market is the heterogeneity of diagnostic performance and the risk of overdiagnosis, with resultant unnecessary treatments. Prostate-specific antigen (PSA) screening tests commonly yield false-positive results or identify clinically insignificant tumors, resulting in unnecessary biopsies, overtreatment, and patient anxiety. Studies reveal that as much as 50% of biopsy-proven prostate cancers are indolent and could never evolve to become lethal. This triggers concerns about over-treatment using surgery or radiotherapy, with attendant side effects of incontinence and impotence. The lack of standardized biomarkers for discriminating between aggressive and non-aggressive cancers is an ongoing challenge. Technological advances in AI-based diagnostic devices, genomic screening, and multiparametric MRI are being investigated to enhance diagnostic accuracy and eliminate unnecessary treatments.
Prostate Cancer Diagnostics Market Segmentation Analysis
By Test Type
The Confirmatory Tests segment dominated the Prostate Cancer Diagnostics Market in 2023 with around 65% market share, because of its pivotal role in conclusive prostate cancer diagnosis. Confirmatory tests like biopsy-based histopathology, advanced imaging (MRI, PET scans), and molecular diagnostics (like PCA3 and 4Kscore tests) have high specificity and accuracy for prostate cancer detection. The increased use of these tests is a result of the increasing prevalence of prostate cancer, progress in biomarker-based testing, and the prevalence of precision medicine. Guidelines by prominent health bodies, including the American Urological Association (AUA) and the National Comprehensive Cancer Network (NCCN), also prioritize confirmatory diagnostics before treatment, which further fuels demand. The segment also saw the adoption of technology, like AI-based imaging and liquid biopsy-based confirmatory tests, which increased diagnostic accuracy and helped the segment lead the market in 2023.
The Preliminary Tests segment is expected to witness the fastest growth in the forecast period because of increasing emphasis on early detection and active screening programs. Initial screenings, including PSA (Prostate-Specific Antigen) assays and digital rectal exams (DRE), are commonly applied as first-line screening tests, especially in general health check-ups for men aged 50 years and above. Growing awareness efforts by healthcare providers, government drives for early detection of cancer, and improvements in PSA-based biomarker testing drive growth. Also, new non-invasive screening technologies, including urine-based biomarker tests and AI-driven risk assessment models, are becoming increasingly popular, further driving the growth of the segment. The transition towards individualized screening approaches and the adoption of multi-omics technologies in the early detection of prostate cancer is likely to drive the fast growth of the Preliminary Tests segment during the forecast period.
By Type
The Adenocarcinoma segment dominated the Prostate Cancer Diagnostics Market in 2023 because of its staggering prevalence among prostate cancer cases. Adenocarcinoma constitutes more than 95% of all diagnosed cases of prostate cancer and is therefore the most prevalent and researched subtype. This huge prevalence has triggered major developments in diagnostic techniques, such as PSA screening, MRI-targeted biopsies, and molecular assays, which are specially developed to identify and determine adenocarcinoma. Moreover, the prevalence of established treatment protocols and specific therapies has added to the requirement for precise and timely diagnosis of this subtype. The extensive adoption of confirmatory tests, including immunohistochemistry (IHC) and adenocarcinoma genetic profiling, combined with enhanced awareness and early screening programs, drove the segment's market leadership in 2023.
The Interstitial Cell Carcinoma segment is anticipated to register the fastest growth during the forecast period owing to its rarity and growing emphasis on enhancing diagnostic tools for less prevalent and aggressive prostate cancer. Interstitial cell carcinoma is a rare and extremely aggressive type of prostate cancer, frequently necessitating specialized diagnostic methods like advanced imaging, next-generation sequencing (NGS), and immunohistochemical markers. With the growth in personalized medicine and precision oncology, there is an increased need for improved characterization of rare types of cancer, resulting in greater research and development activities in this segment. Also, the improvement in liquid biopsy methods and AI-based pathology is improving early detection and identification of rare subtypes of prostate cancer, driving growth in the Interstitial Cell Carcinoma segment. With increasing awareness and clinical studies, this segment will grow at a tremendous pace during the forecast year.
By End Use
The Outpatient Facilities segment dominated the Prostate Cancer Diagnostics Market in 2023 due to the growing favor for minimally invasive diagnostic exams and the higher uptake of budget-friendly, outpatient-based care strategies. Outpatient facilities, such as diagnostic facilities and specialty clinics, provide an easy and time-saving alternative to hospital-based tests, enabling patients to receive prostate cancer screening tests, PSA testing, and biopsies without hospital stay. These centers are staffed with cutting-edge diagnostic technology, including MRI-targeted biopsies and molecular tests, to ensure accuracy in detecting prostate cancer and decrease wait times for patients. Also, the increased focus on early detection and screening for prostate cancer, especially in older populations, has led to more patients opting for outpatient care. The capability to offer quicker, specialized diagnostic services at lower expense than hospitals further entrenched the segment in 2023.
Prostate Cancer Diagnostics Market Regional Insights
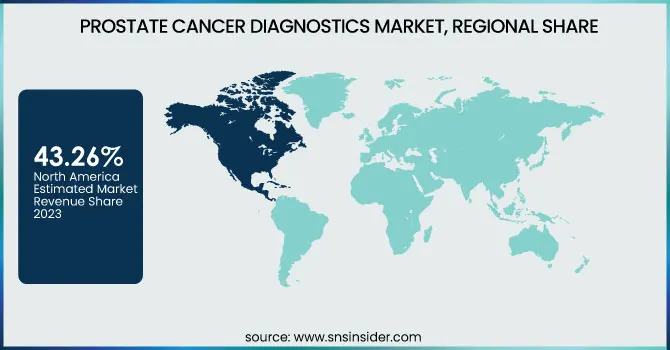
North America dominated the Prostate Cancer Diagnostics market with a 43.26% market share in 2023 because of its sophisticated health infrastructure, superior awareness levels, and aggressive uptake of cutting-edge diagnostic technologies. North America also reports one of the highest rates of prostate cancer globally, with the American Cancer Society estimating more than 288,300 new U.S. cases in 2023 alone. Besides, the presence of major players in the market, including Abbott Laboratories, Roche Diagnostics, and Siemens Healthineers, encourages incessant innovation and access to state-of-the-art diagnostic solutions like PSA tests, MRI-targeted biopsies, and liquid biopsy tests. Encouraging reimbursement policies and government support for cancer research are additional drivers for market growth. Additionally, North America has seen rising adoption of genomic testing and AI-driven diagnostics solutions in prostate cancer, enhancing early diagnosis and tailored treatment planning, further cementing its global market leadership.
The Asia Pacific is the fastest-growing region with a 7.59% CAGR throughout the forecast period, fueled by growing incidence rates of cancer, expanding healthcare spending, and increased development of diagnostic infrastructure. Prostate cancer in Asia, as per WHO, is on the rise with an increase in aging populations, shifting lifestyles, and better cancer screening initiatives. Nations such as China, India, and Japan are observing increased use of PSA testing, biomarker diagnostics, and artificial intelligence-based imaging solutions. Government efforts to increase cancer awareness, screening schemes, and healthcare accessibility further fuel market growth. Also, growing investments by international diagnostic firms and expanding clinical research partnerships in Asia-Pacific are driving technological innovation. The region's transition towards precision medicine and biomarker-based testing is likely to drive demand further, positioning Asia Pacific as the fastest-growing market during the forecast period.
Get Customized Report as per Your Business Requirement - Enquiry Now
Prostate Cancer Diagnostics Market Key Players
-
Abbott Laboratories (ARCHITECT Total PSA Assay, Alinity m System)
-
Siemens Healthineers (ADVIA Centaur XP PSA Assay, MAGNETOM MRI Systems)
-
Roche Diagnostics (Elecsys Total PSA Assay, cobas e 801 Analytical Unit)
-
Hologic, Inc. (Aptima PCA3 Assay, Panther Fusion System)
-
Myriad Genetics, Inc. (Prolaris Test, BRACAnalysis)
-
OPKO Health, Inc. (4Kscore Test, Claros 1 Analyzer)
-
Genomic Health (Exact Sciences Corporation) (Oncotype DX Genomic Prostate Score, Cologuard Test)
-
MDxHealth (SelectMDx, ConfirmMDx)
-
Beckman Coulter, Inc. (Access Hybritech PSA Assay, DxI 800 Immunoassay System)
-
Thermo Fisher Scientific Inc. (Ion Torrent Genexus System, Oncomine Dx Target Test)
-
EDX Medical Group (AI-Driven Prostate Cancer Supertest, Multi-Omics Diagnostic Platform)
-
BioMérieux SA (VIDAS Total PSA Assay, NucliSENS EasyQ System)
-
DiaSorin S.p.A. (LIAISON XL murex PSA Assay, LIAISON MDX)
-
QuidelOrtho Corporation (Triage Total PSA Test, Sofia 2 Fluorescent Immunoassay Analyzer)
-
Becton, Dickinson, and Company (BD) (BD Onclarity HPV Assay, BD MAX System)
-
AstraZeneca plc (Lynparza (Olaparib) Companion Diagnostic, Tagrisso (osimertinib) Companion Diagnostic)
-
Pfizer Inc. (Xtandi (Enzalutamide) Companion Diagnostic, Talzenna (talazoparib) Companion Diagnostic)
-
Novartis AG (Kisqali (ribociclib) Companion Diagnostic, Piqray (Alpelisib) Companion Diagnostic)
-
EDAP TMS (Focal One High-Intensity Focused Ultrasound (HIFU), ExactVu Micro-Ultrasound System)
-
Hitachi, Ltd. (HI VISION Preirus Ultrasound System, EUB-8500 Ultrasound Scanner)
Suppliers (These suppliers play a crucial role in supporting diagnostic companies with essential materials and technologies required for prostate cancer diagnostics.)
-
Merck KGaA
-
Agilent Technologies, Inc.
-
Qiagen N.V.
-
Thermo Fisher Scientific Inc.
-
PerkinElmer Inc.
-
Bio-Rad Laboratories, Inc.
-
Luminex Corporation (DiaSorin Group)
-
Tecan Group Ltd.
-
GE Healthcare
-
R&D Systems (Bio-Techne Corporation)
Recent Development
-
September 2024: Abbott Laboratories gained FDA 510(k) approval for its cutting-edge prostate-specific antigen (PSA) test intended to enhance prostate cancer detection earlier.
-
December 2024: Siemens Healthineers was successful in purchasing the Advanced Accelerator Applications Molecular Imaging unit of Novartis. With this, the ability to generate radioactive tracers essential for positron emission tomography (PET) scans will be bolstered further for Siemens, while increasing its prostate cancer diagnostics offering even more.
-
September 2024: Roche Diagnostics was identified as a top innovator in the worldwide prostate cancer diagnostics market with significant input on the development of diagnostic technologies.
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | US$ 8.57 billion |
| Market Size by 2032 | US$ 15.09 billion |
| CAGR | CAGR of 6.50% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Test Type (Preliminary Tests, Confirmatory Tests) • By Type (Adenocarcinoma, Interstitial Cell Carcinoma, Other) • By End Use (Hospitals, Outpatient Facilities, Home Care, Research & Manufacturing) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | Abbott Laboratories, Siemens Healthineers, Roche Diagnostics, Hologic, Inc., Myriad Genetics, Inc., OPKO Health, Inc., Genomic Health (Exact Sciences Corporation), MDxHealth, Beckman Coulter, Inc., Thermo Fisher Scientific Inc., EDX Medical Group, BioMérieux SA, DiaSorin S.p.A., QuidelOrtho Corporation, Becton, Dickinson and Company (BD), AstraZeneca plc, Pfizer Inc., Novartis AG, EDAP TMS, Hitachi, Ltd., and other players. |

