Get more information on Pharmacovigilance And Drug Safety Software Market - Request Sample Report
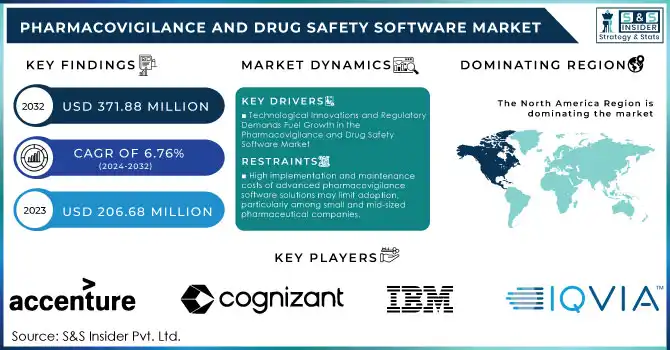
The Pharmacovigilance and Drug Safety Software Market Size was valued at USD 206.68 million in 2023 and is expected to reach USD 371.88 million by 2032 and grow at a CAGR of 6.76% over the forecast period 2024-2032.
The Pharmacovigilance and Drug Safety Software Market has witnessed significant growth, driven by the increasing complexity of drug safety monitoring, regulatory demands, and technological innovations. With more pharmaceutical products entering the market and patient safety becoming a top priority, the need for advanced pharmacovigilance systems to monitor adverse drug reactions (ADRs) has grown substantially. The global pharmaceutical R&D spending in 2022 exceeded USD 200 billion, resulting in a surge of new drugs requiring continuous post-market safety monitoring. This increasing number of drugs entering the market intensifies the need for robust pharmacovigilance solutions to manage ADR data and comply with regulatory guidelines. The FDA's REMS (Risk Evaluation and Mitigation Strategies) program, for instance, requires continuous post-market surveillance for certain high-risk drugs, highlighting the growing need for sophisticated safety systems.
As the industry evolves, AI-driven pharmacovigilance software is transforming drug safety processes. Companies like ArisGlobal have integrated machine learning and natural language processing (NLP) into their platforms, enhancing their ability to detect safety signals and analyze vast amounts of unstructured data from sources like electronic health records, clinical trial data, and social media. AI-powered systems can process millions of patient reports in real time, enabling quicker identification of potential ADRs. For example, Pfizer has successfully employed AI in pharmacovigilance to monitor vaccine safety more effectively, particularly during the rollout of COVID-19 vaccines. The regulatory landscape continues to evolve, with agencies like the FDA and the European Medicines Agency (EMA) regularly updating their pharmacovigilance guidelines. The FDA’s REMS program requires drug manufacturers to assess the risks of new drugs post-approval, and these evolving regulations are pushing pharmaceutical companies to adopt more sophisticated and efficient pharmacovigilance software to ensure compliance. As of 2023, there were over 1.2 million ADR reports filed with the FDA alone, underlining the importance of robust pharmacovigilance systems in managing vast amounts of safety data.
Technological advancements also include the integration of blockchain technology, which is gaining traction due to its ability to provide enhanced data security and transparency. This is especially critical in managing sensitive patient data across multiple stakeholders. For example, Veeva Systems has started incorporating blockchain into its pharmacovigilance solutions to improve traceability and data integrity, addressing the growing concerns over cybersecurity in the healthcare sector. The rise of cloud-based solutions further contributes to market growth. Cloud-based platforms enable pharmaceutical companies to centralize data, collaborate across geographies, and comply with global regulatory requirements more effectively. For instance, Oracle’s pharmacovigilance software leverages the cloud to facilitate real-time ADR reporting, enhancing global safety monitoring capabilities.
Overall, the Pharmacovigilance and Drug Safety Software Market is evolving rapidly, driven by technological advancements like AI, machine learning, and blockchain, as well as increasing regulatory demands. The global pharmaceutical industry’s growing focus on patient safety and compliance is pushing the market toward continuous innovation, ensuring that drug safety processes remain efficient, accurate, and timely.
Drivers
Technological Innovations and Regulatory Demands Fuel Growth in the Pharmacovigilance and Drug Safety Software Market
The growth of the Pharmacovigilance and Drug Safety Software Market is largely driven by several key factors, with increasing regulatory demands and technological innovations leading the way. The global pharmaceutical R&D spending surpassed $200 billion in 2022, which directly correlates to the rise in new drug approvals and the subsequent need for vigilant post-market surveillance. As new drugs enter the market, regulatory agencies like the FDA and EMA mandate ongoing monitoring of adverse drug reactions (ADRs), making pharmacovigilance software essential for compliance. For instance, the FDA reported receiving over 1.2 million ADR reports in 2023, underscoring the vast scale of data that needs to be processed efficiently.
Advancements in AI and machine learning are significantly transforming drug safety processes, driving market growth. AI technologies help companies identify safety signals more accurately by processing millions of data points in real-time. A key example is Pfizer, which uses AI to monitor vaccine safety, especially during high-demand periods like the COVID-19 vaccine rollout. Furthermore, cloud-based pharmacovigilance solutions allow for real-time data access, enabling global collaboration and more streamlined compliance with international regulatory requirements.
The integration of blockchain technology also offers enhanced data security and transparency, a crucial factor as cybersecurity concerns grow within the healthcare sector. These innovations, combined with increasing drug approvals and regulatory scrutiny, are fueling demand for advanced pharmacovigilance software solutions that ensure drug safety and regulatory compliance across the industry.
Restraints
High implementation and maintenance costs of advanced pharmacovigilance software solutions may limit adoption, particularly among small and mid-sized pharmaceutical companies.
Data privacy and security concerns, especially with the integration of cloud and blockchain technologies, pose challenges in ensuring compliance with global data protection regulations.
By Functionality
The Case Data Collection and Management segment led the market in 2023, accounting for approximately 45.0% of the market share. This segment remains critical due to the increasing need for structured and efficient management of adverse drug reactions (ADRs) and other safety reports. The ability to collect, track, and manage safety cases accurately is paramount for compliance with regulatory agencies such as the FDA and EMA. As pharmaceutical companies experience an uptick in drug approvals, there is a rising demand for software solutions that streamline the collection and management of ADR reports, making this segment dominant in 2023.
The Signal Detection and Other Safety Risk Assessment segment is the fastest-growing, driven by the increasing use of AI and machine learning technologies. The need to detect safety signals early through real-time data analysis is crucial for proactive pharmacovigilance, making this functionality a critical tool for pharmaceutical and biotech companies. The segment is projected to grow at a CAGR of 14% in the coming years, as AI and machine learning capabilities advance, allowing more accurate detection of potential risks.
By Delivery Mode
The On-Premise delivery mode dominated the market in 2023, holding around 60.0% of the market share. On-premise solutions provide greater control over sensitive data, which is essential in the pharmacovigilance domain, especially given the regulatory requirements for data security and privacy. Pharmaceutical and biotech companies often prefer on-premise software for enhanced data security, customization, and integration with existing systems. Additionally, the demand for long-term data storage and control over proprietary information has kept on-premise solutions as the preferred choice in the industry.
The On-Demand delivery model is the fastest-growing segment, driven by the increasing shift toward cloud-based solutions. On-demand solutions offer scalability, flexibility, and lower upfront costs, making them particularly attractive to small and mid-sized pharmaceutical companies and contract research organizations (CROs). This segment is expected to grow at a CAGR of 20% as more companies embrace cloud solutions for better collaboration, real-time data access, and global regulatory compliance.
By End-use
The Pharma & Biotech Companies segment held the largest market share in 2023, comprising approximately 55.0% of the market. These companies are the primary drivers of demand for pharmacovigilance and drug safety software, as they are directly responsible for the development, approval, and monitoring of new drugs. The ongoing regulatory scrutiny and the increasing number of drug approvals necessitate robust pharmacovigilance systems, making pharma and biotech companies the dominant players in the market.
The Contract Research Organizations (CROs) segment is the fastest-growing end-user segment, with a projected CAGR of 16%. As CROs increasingly manage clinical trials and post-market surveillance for multiple pharmaceutical companies, the demand for efficient pharmacovigilance solutions grows. CROs benefit from cloud-based solutions and AI-driven risk assessments, allowing them to handle larger volumes of data across diverse clients. The trend toward outsourcing clinical trials and drug safety monitoring to CROs is a key driver of growth in this segment.
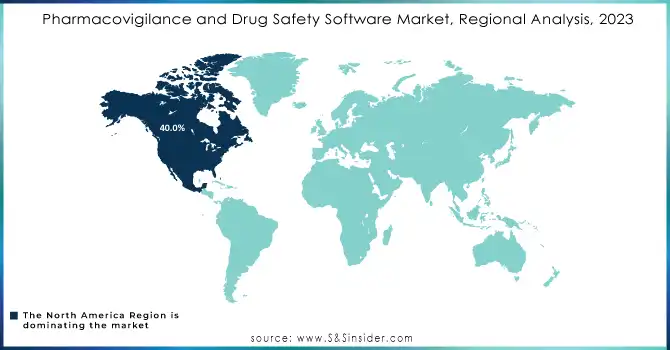
North America dominated the Pharmacovigilance and Drug Safety Software Market in 2023, accounting for approximately 40.0% of the market share. This dominance is attributed to the presence of key pharmaceutical and biotechnology companies, along with stringent regulatory requirements set by the FDA and other health authorities. The U.S. continues to lead in pharmaceutical R&D spending, which fuels the demand for advanced drug safety solutions. Additionally, the adoption of AI, machine learning, and cloud-based solutions has accelerated the growth of pharmacovigilance software in this region.
Europe held the second-largest market share, with the European Medicines Agency (EMA) enforcing strict pharmacovigilance regulations that drive software adoption. The market in Europe is characterized by a strong focus on data privacy and security, as well as increasing collaboration between pharmaceutical companies and regulatory bodies. The UK and Germany are the leading markets, accounting for a significant portion of the regional share.
Asia-Pacific is the fastest-growing region, projected to experience a CAGR of 17% in the coming years. Rapid growth in pharmaceutical R&D, rising healthcare spending, and the expansion of the pharmaceutical industry in countries like China and India contribute to this growth. Additionally, the increasing adoption of cloud-based pharmacovigilance software and AI technologies in the region is driving the demand for drug safety solutions.
Need any customization research on Pharmacovigilance and Drug Safety Software Market - Enquiry Now
1. IQVIA
IQVIA Vigilance
Pharmacovigilance Platform
Signal Detection and Risk Management Solutions
2. Accenture
Accenture Pharmacovigilance Solutions
Cloud-based Safety Solutions
Digital Transformation Services
3. Cognizant
Cognizant’s Drug Safety and Pharmacovigilance Services
Safety Data Management Solutions
4. Laboratory Corporation of America Holdings (LabCorp)
LabCorp Drug Safety Services
Pharmacovigilance Case Management Solutions
5. IBM
IBM Watson for Drug Safety
AI-driven Pharmacovigilance Solutions
6. ArisGlobal
ArisGlobal LifeSphere Safety
LifeSphere Pharmacovigilance Suite
7. ICON Plc.
ICON Pharmacovigilance Solutions
Safety and Risk Management Services
8. Capgemini
Capgemini Pharmacovigilance Services
AI-driven Safety Monitoring Solutions
9. Oracle
Oracle Argus Safety
Oracle Health Sciences Safety Solutions
10. Parexel International Corporation
Parexel Pharmacovigilance Services
Safety Data Management and Risk Assessment Solutions
11. ArisEurope
LifeSphere Safety Solutions
Pharmacovigilance Suite
12. Syneos Health
Syneos Health Safety and Pharmacovigilance Solutions
Drug Safety Case Management Services
13. Genpact
Genpact Pharmacovigilance Services
Safety Monitoring and Risk Assessment Solutions
14. Max Application
Max Pharmacovigilance Solutions
Adverse Event Reporting and Management Software
In November 2024, IQVIA announced its plan to cut pharmacovigilance costs by 50% while achieving over 99% accuracy using generative AI. Uwe Trinks, Global Practice Lead of Pharmacovigilance Technologies, highlighted that this AI-driven approach will reduce human verification efforts, improving data quality significantly compared to traditional methods.
In June 2024, the FDA's Center for Drug Evaluation and Research (CDER) announced the launch of the Emerging Drug Safety Technology Meeting (EDSTM) program. This initiative, part of CDER's Emerging Drug Safety Technology Program, will facilitate discussions between stakeholders in pharmacovigilance and CDER staff on the role of artificial intelligence in advancing drug safety practices.
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | US$ 206.68 billion |
| Market Size by 2032 | US$ 371.88 billion |
| CAGR | CAGR of 6.76% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Functionality (Case data collection and management, Signal detection and other safety risk assessment, Safety Metrics & Others) • By Delivery Mode (On-Premise, On-Demand) • By End-use (Pharma & Biotech Companies, CRO, BPO, Others) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | IQVIA, Accenture, Cognizant, Laboratory Corporation of America Holdings (LabCorp), IBM, ArisGlobal, ICON Plc., Capgemini, Oracle, Parexel International Corporation, ArisEurope, Syneos Health, Genpact, Max Application. |
| Key Drivers | • Technological Innovations and Regulatory Demands Fuel Growth in the Pharmacovigilance and Drug Safety Software Market |
| Restraints | • High implementation and maintenance costs of advanced pharmacovigilance software solutions may limit adoption, particularly among small and mid-sized pharmaceutical companies. • Data privacy and security concerns, especially with the integration of cloud and blockchain technologies, pose challenges in ensuring compliance with global data protection regulations. |
Ans: The Pharmacovigilance and Drug Safety Software market is expected to grow at a CAGR of 6.76% over the forecast period 2024-2032.
Pharmacovigilance is not widely known are the restraints of the market.
Pharmacovigilance and Drug Safety Software Market is divided into three segments and they are By Functionality, By Delivery, and By End-User
The market for pharmacovigilance and drug safety software is anticipated to be dominated by North America.
Ans: The Pharmacovigilance and Drug Safety Software Market size was valued at US$ 206.68 million n 2023.
Table of Contents
1. Introduction
1.1 Market Definition
1.2 Scope (Inclusion and Exclusions)
1.3 Research Assumptions
2. Executive Summary
2.1 Market Overview
2.2 Regional Synopsis
2.3 Competitive Summary
3. Research Methodology
3.1 Top-Down Approach
3.2 Bottom-up Approach
3.3. Data Validation
3.4 Primary Interviews
4. Market Dynamics Impact Analysis
4.1 Market Driving Factors Analysis
4.1.1 Drivers
4.1.2 Restraints
4.1.3 Opportunities
4.1.4 Challenges
4.2 PESTLE Analysis
4.3 Porter’s Five Forces Model
5. Statistical Insights and Trends Reporting
5.1 Incidence and Prevalence (2023)
5.2 Prescription Trends (2023), by Region
5.3 Device Volume, by Region (2020-2032)
5.4 Healthcare Spending, by Region (Government, Commercial, Private, Out-of-Pocket), 2023
5.5 Growth of AI and Machine Learning Technologies
5.6 Regulatory Impact on Software Usage
6. Competitive Landscape
6.1 List of Major Companies, By Region
6.2 Market Share Analysis, By Region
6.3 Product Benchmarking
6.3.1 Product specifications and features
6.3.2 Pricing
6.4 Strategic Initiatives
6.4.1 Marketing and promotional activities
6.4.2 Distribution and Supply Chain Strategies
6.4.3 Expansion plans and new Product launches
6.4.4 Strategic partnerships and collaborations
6.5 Technological Advancements
6.6 Market Positioning and Branding
7. Pharmacovigilance and Drug Safety Software Market Segmentation, by Functionality
7.1 Chapter Overview
7.2 Case data collection and management
7.2.1 Case data collection and management Market Trends Analysis (2020-2032)
7.2.2 Case data collection and management Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3 Signal detection and other safety risk assessment
7.3.1 Signal detection and other safety risk assessment Market Trends Analysis (2020-2032)
7.3.2 Signal detection and other safety risk assessment Market Size Estimates and Forecasts to 2032 (USD Billion)
7.4 Safety Metrics & Others
7.4.1 Safety Metrics & Others Market Trends Analysis (2020-2032)
7.4.2 Safety Metrics & Others Market Size Estimates and Forecasts to 2032 (USD Billion)
8. Pharmacovigilance and Drug Safety Software Market Segmentation, by Delivery Mode
8.1 Chapter Overview
8.2 On-Premise
8.2.1 On-Premise Market Trends Analysis (2020-2032)
8.2.2 On-Premise Market Size Estimates and Forecasts to 2032 (USD Million)
8.3 On-Demand
8.3.1 On-Demand Market Trends Analysis (2020-2032)
8.3.2 On-Demand Market Size Estimates and Forecasts to 2032 (USD Million)
9. Pharmacovigilance and Drug Safety Software Market Segmentation, by End-use
9.1 Chapter Overview
9.2 Pharma & Biotech Companies
9.2.1 Pharma & Biotech Companies Market Trends Analysis (2020-2032)
9.2.2 Pharma & Biotech Companies Market Size Estimates and Forecasts to 2032 (USD Million)
9.3 CRO
9.3.1 CRO Market Trends Analysis (2020-2032)
9.3.2 CRO Market Size Estimates and Forecasts to 2032 (USD Million)
9.4 BPO
9.4.1 BPO Market Trends Analysis (2020-2032)
9.4.2 BPO Market Size Estimates and Forecasts to 2032 (USD Million)
9.5 Others
9.5.1 Others Market Trends Analysis (2020-2032)
9.5.2 Others Market Size Estimates and Forecasts to 2032 (USD Million)
10. Regional Analysis
10.1 Chapter Overview
10.2 North America
10.2.1 Trends Analysis
10.2.2 North America Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Country (2020-2032) (USD Million)
10.2.3 North America Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.2.4 North America Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.2.5 North America Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.2.6 USA
10.2.6.1 USA Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.2.6.2 USA Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.2.6.3 USA Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.2.7 Canada
10.2.7.1 Canada Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.2.7.2 Canada Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.2.7.3 Canada Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.2.8 Mexico
10.2.8.1 Mexico Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.2.8.2 Mexico Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.2.8.3 Mexico Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.3 Europe
10.3.1 Eastern Europe
10.3.1.1 Trends Analysis
10.3.1.2 Eastern Europe Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Country (2020-2032) (USD Million)
10.3.1.3 Eastern Europe Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.3.1.4 Eastern Europe Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.3.1.5 Eastern Europe Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.3.1.6 Poland
10.3.1.6.1 Poland Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.3.1.6.2 Poland Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.3.1.6.3 Poland Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.3.1.7 Romania
10.3.1.7.1 Romania Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.3.1.7.2 Romania Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.3.1.7.3 Romania Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.3.1.8 Hungary
10.3.1.8.1 Hungary Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.3.1.8.2 Hungary Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.3.1.8.3 Hungary Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.3.1.9 turkey
10.3.1.9.1 Turkey Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.3.1.9.2 Turkey Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.3.1.9.3 Turkey Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.3.1.10 Rest of Eastern Europe
10.3.1.10.1 Rest of Eastern Europe Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.3.1.10.2 Rest of Eastern Europe Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.3.1.10.3 Rest of Eastern Europe Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.3.2 Western Europe
10.3.2.1 Trends Analysis
10.3.2.2 Western Europe Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Country (2020-2032) (USD Million)
10.3.2.3 Western Europe Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.3.2.4 Western Europe Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.3.2.5 Western Europe Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.3.2.6 Germany
10.3.2.6.1 Germany Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.3.2.6.2 Germany Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.3.2.6.3 Germany Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.3.2.7 France
10.3.2.7.1 France Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.3.2.7.2 France Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.3.2.7.3 France Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.3.2.8 UK
10.3.2.8.1 UK Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.3.2.8.2 UK Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.3.2.8.3 UK Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.3.2.9 Italy
10.3.2.9.1 Italy Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.3.2.9.2 Italy Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.3.2.9.3 Italy Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.3.2.10 Spain
10.3.2.10.1 Spain Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.3.2.10.2 Spain Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.3.2.10.3 Spain Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.3.2.11 Netherlands
10.3.2.11.1 Netherlands Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.3.2.11.2 Netherlands Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.3.2.11.3 Netherlands Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.3.2.12 Switzerland
10.3.2.12.1 Switzerland Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.3.2.12.2 Switzerland Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.3.2.12.3 Switzerland Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.3.2.13 Austria
10.3.2.13.1 Austria Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.3.2.13.2 Austria Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.3.2.13.3 Austria Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.3.2.14 Rest of Western Europe
10.3.2.14.1 Rest of Western Europe Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.3.2.14.2 Rest of Western Europe Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.3.2.14.3 Rest of Western Europe Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.4 Asia Pacific
10.4.1 Trends Analysis
10.4.2 Asia Pacific Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Country (2020-2032) (USD Million)
10.4.3 Asia Pacific Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.4.4 Asia Pacific Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.4.5 Asia Pacific Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.4.6 China
10.4.6.1 China Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.4.6.2 China Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.4.6.3 China Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.4.7 India
10.4.7.1 India Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.4.7.2 India Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.4.7.3 India Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.4.8 Japan
10.4.8.1 Japan Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.4.8.2 Japan Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.4.8.3 Japan Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.4.9 South Korea
10.4.9.1 South Korea Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.4.9.2 South Korea Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.4.9.3 South Korea Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.4.10 Vietnam
10.4.10.1 Vietnam Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.4.10.2 Vietnam Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.4.10.3 Vietnam Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.4.11 Singapore
10.4.11.1 Singapore Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.4.11.2 Singapore Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.4.11.3 Singapore Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.4.12 Australia
10.4.12.1 Australia Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.4.12.2 Australia Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.4.12.3 Australia Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.4.13 Rest of Asia Pacific
10.4.13.1 Rest of Asia Pacific Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.4.13.2 Rest of Asia Pacific Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.4.13.3 Rest of Asia Pacific Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.5 Middle East and Africa
10.5.1 Middle East
10.5.1.1 Trends Analysis
10.5.1.2 Middle East Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Country (2020-2032) (USD Million)
10.5.1.3 Middle East Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.5.1.4 Middle East Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.5.1.5 Middle East Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.5.1.6 UAE
10.5.1.6.1 UAE Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.5.1.6.2 UAE Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.5.1.6.3 UAE Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.5.1.7 Egypt
10.5.1.7.1 Egypt Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.5.1.7.2 Egypt Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.5.1.7.3 Egypt Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.5.1.8 Saudi Arabia
10.5.1.8.1 Saudi Arabia Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.5.1.8.2 Saudi Arabia Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.5.1.8.3 Saudi Arabia Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.5.1.9 Qatar
10.5.1.9.1 Qatar Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.5.1.9.2 Qatar Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.5.1.9.3 Qatar Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.5.1.10 Rest of Middle East
10.5.1.10.1 Rest of Middle East Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.5.1.10.2 Rest of Middle East Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.5.1.10.3 Rest of Middle East Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.5.2 Africa
10.5.2.1 Trends Analysis
10.5.2.2 Africa Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Country (2020-2032) (USD Million)
10.5.2.3 Africa Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.5.2.4 Africa Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.5.2.5 Africa Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.5.2.6 South Africa
10.5.2.6.1 South Africa Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.5.2.6.2 South Africa Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.5.2.6.3 South Africa Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.5.2.7 Nigeria
10.5.2.7.1 Nigeria Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.5.2.7.2 Nigeria Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.5.2.7.3 Nigeria Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.5.2.8 Rest of Africa
10.5.2.8.1 Rest of Africa Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.5.2.8.2 Rest of Africa Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.5.2.8.3 Rest of Africa Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.6 Latin America
10.6.1 Trends Analysis
10.6.2 Latin America Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Country (2020-2032) (USD Million)
10.6.3 Latin America Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.6.4 Latin America Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.6.5 Latin America Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.6.6 Brazil
10.6.6.1 Brazil Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.6.6.2 Brazil Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.6.6.3 Brazil Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.6.7 Argentina
10.6.7.1 Argentina Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.6.7.2 Argentina Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.6.7.3 Argentina Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.6.8 Colombia
10.6.8.1 Colombia Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.6.8.2 Colombia Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.6.8.3 Colombia Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10.6.9 Rest of Latin America
10.6.9.1 Rest of Latin America Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Functionality (2020-2032) (USD Million)
10.6.9.2 Rest of Latin America Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by Delivery Mode (2020-2032) (USD Million)
10.6.9.3 Rest of Latin America Pharmacovigilance and Drug Safety Software Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
11. Company Profiles
11.1 IQVIA
11.1.1 Company Overview
11.1.2 Financial
11.1.3 Products/ Services Offered
11.1.4 SWOT Analysis
11.2 Accenture
11.2.1 Company Overview
11.2.2 Financial
11.2.3 Products/ Services Offered
11.2.4 SWOT Analysis
11.3 Cognizant
11.3.1 Company Overview
11.3.2 Financial
11.3.3 Products/ Services Offered
11.3.4 SWOT Analysis
11.4 Laboratory Corporation of America Holdings (LabCorp)
11.4.1 Company Overview
11.4.2 Financial
11.4.3 Products/ Services Offered
11.4.4 SWOT Analysis
11.5 IBM
11.5.1 Company Overview
11.5.2 Financial
11.5.3 Products/ Services Offered
11.5.4 SWOT Analysis
11.6 ArisGlobal
11.6.1 Company Overview
11.6.2 Financial
11.6.3 Products/ Services Offered
11.6.4 SWOT Analysis
11.7 ICON Plc.
11.7.1 Company Overview
11.7.2 Financial
11.7.3 Products/ Services Offered
11.7.4 SWOT Analysis
11.8 Capgemini
11.8.1 Company Overview
11.8.2 Financial
11.8.3 Products/ Services Offered
11.8.4 SWOT Analysis
11.9 Oracle
11.9.1 Company Overview
11.9.2 Financial
11.9.3 Products/ Services Offered
11.9.4 SWOT Analysis
11.10 Parexel International Corporation
11.10.1 Company Overview
11.10.2 Financial
11.10.3 Products/ Services Offered
11.10.4 SWOT Analysis
12. Use Cases and Best Practices
13. Conclusion
An accurate research report requires proper strategizing as well as implementation. There are multiple factors involved in the completion of good and accurate research report and selecting the best methodology to compete the research is the toughest part. Since the research reports we provide play a crucial role in any company’s decision-making process, therefore we at SNS Insider always believe that we should choose the best method which gives us results closer to reality. This allows us to reach at a stage wherein we can provide our clients best and accurate investment to output ratio.
Each report that we prepare takes a timeframe of 350-400 business hours for production. Starting from the selection of titles through a couple of in-depth brain storming session to the final QC process before uploading our titles on our website we dedicate around 350 working hours. The titles are selected based on their current market cap and the foreseen CAGR and growth.
The 5 steps process:
Step 1: Secondary Research:
Secondary Research or Desk Research is as the name suggests is a research process wherein, we collect data through the readily available information. In this process we use various paid and unpaid databases which our team has access to and gather data through the same. This includes examining of listed companies’ annual reports, Journals, SEC filling etc. Apart from this our team has access to various associations across the globe across different industries. Lastly, we have exchange relationships with various university as well as individual libraries.

Step 2: Primary Research
When we talk about primary research, it is a type of study in which the researchers collect relevant data samples directly, rather than relying on previously collected data. This type of research is focused on gaining content specific facts that can be sued to solve specific problems. Since the collected data is fresh and first hand therefore it makes the study more accurate and genuine.
We at SNS Insider have divided Primary Research into 2 parts.
Part 1 wherein we interview the KOLs of major players as well as the upcoming ones across various geographic regions. This allows us to have their view over the market scenario and acts as an important tool to come closer to the accurate market numbers. As many as 45 paid and unpaid primary interviews are taken from both the demand and supply side of the industry to make sure we land at an accurate judgement and analysis of the market.
This step involves the triangulation of data wherein our team analyses the interview transcripts, online survey responses and observation of on filed participants. The below mentioned chart should give a better understanding of the part 1 of the primary interview.

Part 2: In this part of primary research the data collected via secondary research and the part 1 of the primary research is validated with the interviews from individual consultants and subject matter experts.
Consultants are those set of people who have at least 12 years of experience and expertise within the industry whereas Subject Matter Experts are those with at least 15 years of experience behind their back within the same space. The data with the help of two main processes i.e., FGDs (Focused Group Discussions) and IDs (Individual Discussions). This gives us a 3rd party nonbiased primary view of the market scenario making it a more dependable one while collation of the data pointers.
Step 3: Data Bank Validation
Once all the information is collected via primary and secondary sources, we run that information for data validation. At our intelligence centre our research heads track a lot of information related to the market which includes the quarterly reports, the daily stock prices, and other relevant information. Our data bank server gets updated every fortnight and that is how the information which we collected using our primary and secondary information is revalidated in real time.

Step 4: QA/QC Process
After all the data collection and validation our team does a final level of quality check and quality assurance to get rid of any unwanted or undesired mistakes. This might include but not limited to getting rid of the any typos, duplication of numbers or missing of any important information. The people involved in this process include technical content writers, research heads and graphics people. Once this process is completed the title gets uploader on our platform for our clients to read it.
Step 5: Final QC/QA Process:
This is the last process and comes when the client has ordered the study. In this process a final QA/QC is done before the study is emailed to the client. Since we believe in giving our clients a good experience of our research studies, therefore, to make sure that we do not lack at our end in any way humanly possible we do a final round of quality check and then dispatch the study to the client.
By Functionality
Case data collection and management
Signal detection and other safety risk assessment
Safety Metrics & Others
By Delivery Mode
On-Premise
On-Demand
By End-use
Pharma & Biotech Companies
CRO
BPO
Others
Request for Segment Customization as per your Business Requirement: Segment Customization Request
Regional Coverage:
North America
US
Canada
Mexico
Europe
Eastern Europe
Poland
Romania
Hungary
Turkey
Rest of Eastern Europe
Western Europe
Germany
France
UK
Italy
Spain
Netherlands
Switzerland
Austria
Rest of Western Europe
Asia Pacific
China
India
Japan
South Korea
Vietnam
Singapore
Australia
Rest of Asia Pacific
Middle East & Africa
Middle East
UAE
Egypt
Saudi Arabia
Qatar
Rest of the Middle East
Africa
Nigeria
South Africa
Rest of Africa
Latin America
Brazil
Argentina
Colombia
Rest of Latin America
Request for Country Level Research Report: Country Level Customization Request
Available Customization
With the given market data, SNS Insider offers customization as per the company’s specific needs. The following customization options are available for the report:
Product Analysis
Criss-Cross segment analysis (e.g. Product X Application)
Product Matrix which gives a detailed comparison of the product portfolio of each company
Geographic Analysis
Additional countries in any of the regions
Company Information
Detailed analysis and profiling of additional market players (Up to five)
The Orthopedic Joint Replacement Market size was estimated at USD 22.45 billion in 2023 and is expected to reach USD 44.87billion by 2032 at a CAGR of 8% during the forecast period of 2024-2032.
The Healthcare Middleware Market Size was valued at USD 3.0 Billion in 2023 and is expected to reach USD 7.06 Billion by 2032 and grow at a CAGR of 9.97% over the forecast period 2024-2032.
The Patient Handling Equipment Market size was estimated at USD 10.94 billion in 2023 and is expected to reach USD 17.85 billion By 2031 at a CAGR of 6.3% during the forecast period of 2024-2031.
The Diabetic Nephropathy Market size was valued at USD 2.33 Billion in 2023 and is expected to reach USD 3.72 Billion By 2031 and grow at a CAGR of 6.04% over the forecast period of 2024-2031.
The Intraoral Cameras Market size was valued at USD 2.2 billion in 2023 and is expected to reach USD 5.8 billion by 2032 and grow at a CAGR of 11.4% over the forecast period 2024-2032.
The Prescription Drugs market size was USD 1162.60 billion in 2023 and is expected to reach USD 2173.61 billion by 2032 and grow at a CAGR of 7.20% over the forecast period of 2024-2032.
Hi! Click one of our member below to chat on Phone