Get more information on Pharmaceutical Sterility Testing Market - Request Sample Report
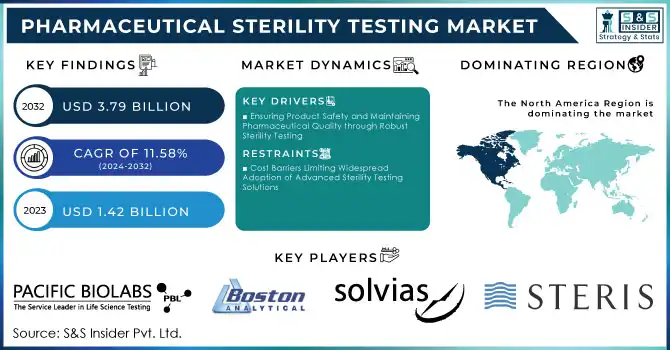
Pharmaceutical Sterility Testing Market was valued at USD 1.42 billion in 2023 and is expected to reach USD 3.79 billion by 2032, growing at a CAGR of 11.58% from 2024-2032.
The pharmaceutical sterility testing market is expected to exhibit significant growth with a rise in demand for safe and effective pharmaceutical products. The stringent regulations imposed by health authorities such as the FDA and EMA have made pharmaceutical companies ensure that their products remain sterile and safe for patients. Increasing levels of infectious diseases and a pharmaceutical manufacturing industry expanding at an unbelievable rate will continue to propel the demand for sterility testing services. Furthermore, advancement in testing technology, such as automated methods and rapid sterility testing, is expected to spur growth in this market.
The higher growth potential of biopharmaceuticals, such as biologics and gene therapies, is an opening for fast growth in the sterility testing market. With biologics, which require highly sensitive testing to ensure they are free from microbial contamination, ensuring their safety is crucial for the health and well-being of the patient. As the trend for personalized medicine and advanced biologic therapies continues on an upward trajectory, there will be a commensurate increase in sterility testing that calls for precision and reliability. With further growth in this sector, R&D investment in new testing solutions and technologies for these needs is expected to continue to intensify.
The pharmaceutical sterility testing market will expand with the progression of regulation and the rising importance of quality control in pharmaceutical production. The importance of achieving and maintaining sterility at every stage of the manufacturing process, from raw materials to finished products, has placed a huge demand on testing solutions. With increasing biological drugs in the market and emerging therapies that require high sterility controls, the market has huge future potential. Pharmaceutical manufacturing activities are witnessing an increasing trend, and advanced sterility testing of production processes will create further long-term opportunities for established players and new entrants in the market.
DRIVERS
Ensuring Product Safety and Maintaining Pharmaceutical Quality through Robust Sterility Testing
The pharmaceutical sterility testing market addresses the critical need to ensure that injectable drugs, vaccines, and implantable devices do not harbor any microbial contamination that could cause severe patient infections or complications. Testing during the production and packaging processes must be stringent to uphold the optimum levels of product safety. Continuous monitoring thus allows for the maintenance of quality, integrity, and efficacy of pharmaceutical products in support of public health expectations and regulatory requirements. Improved sterility assurance contributes to reducing the risk of contamination directly and maintains a higher level of confidence in pharmaceutical products.
Revolutionizing Sterility Testing with Rapid Detection and Automation Innovations
Sterility testing is advancing due to innovations in testing methods, moving away from conventional techniques such as direct inoculation and membrane filtration toward quicker, more effective strategies. Rapid microbial detection methods, such as PCR testing and ATP bioluminescence, are becoming more prominent due to their capacity to greatly shorten testing durations. Moreover, advancements in automation and real-time monitoring are improving the accuracy and effectiveness of sterility testing, reducing human mistakes. These advancements are enhancing throughput and guaranteeing quicker, more dependable outcomes. The growing need for faster and more precise sterility assurance is a primary market catalyst.
RESTRAINTS
Cost Barriers Limiting Widespread Adoption of Advanced Sterility Testing Solutions
The high cost related to the implementation of rapid microbial detection techniques and automated sterility testing systems represents a major obstacle to market expansion. These technologies typically require a substantial initial investment and ongoing maintenance costs, making them less accessible to smaller pharmaceutical companies or those situated in regions with limited infrastructure. Consequently, the extensive adoption of these innovative testing solutions is limited, especially within budget-conscious companies. The cost of implementing these innovations might restrict their accessibility, hindering widespread market adoption.
BY TYPE
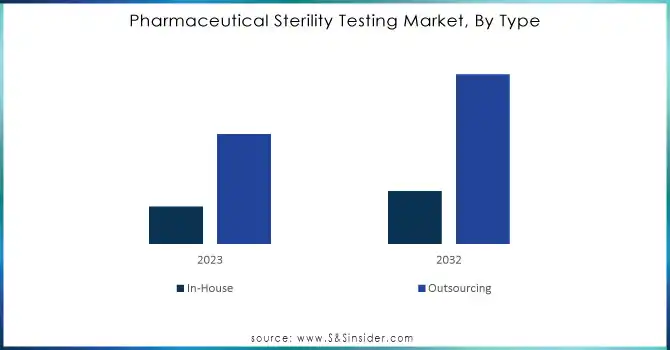
Outsourcing dominated the pharmaceutical sterility testing market in 2023, with the highest share of revenue about 59% with third-party contract research organizations (CROs) and contract manufacturing organizations (CMOs) due to their repute for cost-effectiveness and specialized expertise. Companies prefer outsourcing by reducing the cost of operations, accessing high-specialized testing facilities, and also streamlining the supply chain.
In-house sterility testing is projected to increase at the fastest CAGR of 12.47% during the period from 2024 to 2032, as more companies invest in internal testing capabilities for better quality control, to meet arising regulatory requirements better, and to shorten time-to-market for new drug products. These developments are spurred by advances in the domain of automation and AI, thus making in-house testing much more viable and efficient for most companies.
Need any customization research on Pharmaceutical Sterility Testing Market - Enquiry Now
BY PRODUCT TYPE
Kits and reagents proved to be the largest revenue-generating product type in pharmaceutical sterility testing during 2023, holding a share of above 59%, as it is considered a fundamental product to carry out proper sterility testing. Its products are extensively used in every testing method, which leads to the resulting outcome along with strict regulatory compliance.
The services segment is expected to experience the fastest CAGR of 13.26% from 2024-2032 due to the increasing demand for pharmaceutical companies outsourcing testing services. Service providers decrease pharmaceutical companies' expenses, increase efficiency, and allow pharmaceutical companies access to cutting-edge testing technologies while sparing them the heavy upfront investments in equipment. This trend toward outsourcing sterility testing services will continue to be driven significantly by further scaling up of the industry in the next few years, keeping the focus on a larger production size with stringent quality standards.
BY END USE
In 2023, pharmaceutical companies dominated the pharmaceutical sterility testing market, contributing approximately 43% of the overall revenue. This supremacy is linked to their production of substantial quantities of sterile medications, including injectables and biologics, which undergo strict testing before market release. These firms must comply with stringent regulatory requirements for safety and quality, driving the increasing demand for efficient sterility testing solutions.
The compounding pharmacies are expected to grow at the highest CAGR of 13.47% from 2024 to 2032, considering that the growing demand for individualized medications and customized sterile formulations accelerates. Compounding pharmacies are becoming more invested in sterility testing as tailored treatments increase, particularly in treatment areas such as oncology and hormone therapy.
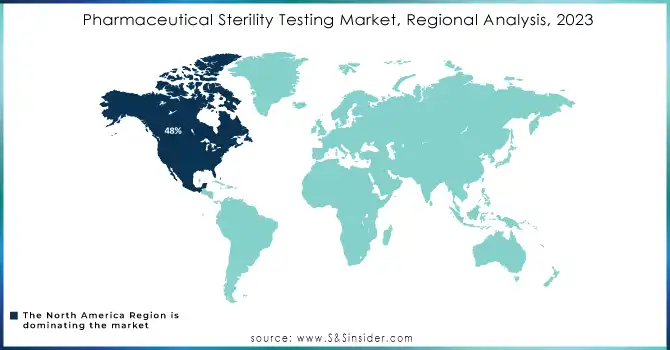
North America held about 48% of market revenue in 2023 due to the region hosting a well-established pharmaceutical industry and having strict regulatory standards. Hosting some of the top pharmaceutical companies, with a focus on advanced drug development, biologics, and injectables, creates tremendous demand for sterility testing services. In addition, the FDA and other similar regulatory authorities have strict regulations on sterility testing, which supports the majority share in this market region.
The Asia Pacific region is anticipated to experience the highest CAGR of 14.58% throughout the forecast period from 2024 to 2032, fueled by swift advancements in pharmaceutical production and rising investments in healthcare facilities. Elements like the increasing need for generic medications and biologics, combined with the expanding count of Contract Manufacturing Organizations (CMOs) in China and India, are fueling this growth. The Asia Pacific's rise as a central location for drug production will boost market growth, leading to a greater need for dependable and effective sterility testing solutions that comply with international quality standards.
LATEST NEWS-
In 2024, Sartorius launched the Sterisart Universal | Gen 4 sterility testing pump, offering advanced features like real-time data sharing and secure electronic documentation to enhance pharmaceutical testing workflows
STERIS introduced Verafit Sterilization Bags and Covers in March 2024, designed to support EU GMP Annex 1 compliance and reduce contamination risks in pharmaceutical sterilization processes
KEY PLAYERS
Pacific Biolabs (Bioburden Testing, Sterility Testing Services)
Steris Plc (Sterility Assurance Products, Rapid Microbial Testing Solutions)
Boston Analytical (Bioburden Testing, Sterility Testing Services)
Sotera Health Company (Nelson Labs) (Sterility Testing, Bioburden Testing)
Sartorius Ag (Sterility Testing Equipment, Filtration Systems for Sterile Applications)
Solvias Ag (Sterility Testing Services, Microbial Testing Solutions)
SGS SA (Sterility Testing Services, Bioburden Testing Solutions)
Labcorp (Sterility Testing, Microbial Limits Testing)
Pace Analytical (Microbial Testing, Sterility Testing Services)
Charles River Laboratories (Sterility Testing, Bioburden Testing)
Thermo Fisher Scientific, Inc. (Sterility Testing Equipment, Microbial Identification Systems)
Rapid Micro Biosystems, Inc. (Automated Microbial Detection System, Rapid Sterility Testing Solutions)
Almac Group (Sterility Testing, Microbial Testing Services)
Labor LS SE & Co. KG (Sterility Testing, Microbial Contamination Testing)
BioMerieux (Sterility Testing Solutions, Microbial Identification Systems)
Lonza Group (Sterility Testing Services, Bioburden Testing)
WuXi AppTec (Sterility Testing Services, Bioburden Testing Solutions)
Merck KGaA (Sterility Testing Reagents, Rapid Microbial Detection Systems)
Eurofins Scientific (Sterility Testing, Bioburden Testing Services)
Pall Corporation (Sterilizing Filtration Systems, Sterility Testing Solutions)
MilliporeSigma (Sterility Testing Kits, Microbial Testing Services)
Viral Inactivation Technologies (Sterility Testing, Viral Clearance Services)
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | USD 1.42 Billion |
| Market Size by 2032 | USD 3.79 Billion |
| CAGR | CAGR of 11.58% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Type (In-House, Outsourcing) • By Product Type (Kits & Reagents, Instruments, Services) • By End-use (Compounding Pharmacies, Medical Device Companies, Pharmaceutical Companies) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | Pacific Biolabs, Steris Plc, Boston Analytical, Sotera Health Company (Nelson Labs), Sartorius Ag, Solvias Ag, SGS SA, Labcorp, Pace Analytical, Charles River Laboratories, Thermo Fisher Scientific, Inc., Rapid Micro Biosystems, Inc., Almac Group, Labor LS SE & Co. KG, BioMerieux, Lonza Group, WuXi AppTec, Merck KGaA, Eurofins Scientific, Pall Corporation, MilliporeSigma, Viral Inactivation Technologies |
| Key Drivers | • Ensuring Product Safety and Maintaining Pharmaceutical Quality through Robust Sterility Testing • Revolutionizing Sterility Testing with Rapid Detection and Automation Innovations |
| RESTRAINTS | • Cost Barriers Limiting Widespread Adoption of Advanced Sterility Testing Solutions |
Pharmaceutical Sterility Testing Market was valued at USD 1.42 billion in 2023 and is expected to reach USD 3.79 billion by 2032, growing at a CAGR of 11.58% from 2024-2032.
Increased R&D in pharmaceuticals, high wholesale turnover, and the rising demand for biologics and injectables are key factors.
North America dominates the market with about 48% of the revenue share.
In-house sterility testing is expected to grow at the highest CAGR of 12.47% due to advances in automation and AI technologies.
Asia Pacific is expected to grow at the highest CAGR of 14.58% from 2024-2032.
Table of Contents:
1. Introduction
1.1 Market Definition
1.2 Scope (Inclusion and Exclusions)
1.3 Research Assumptions
2. Executive Summary
2.1 Market Overview
2.2 Regional Synopsis
2.3 Competitive Summary
3. Research Methodology
3.1 Top-Down Approach
3.2 Bottom-up Approach
3.3. Data Validation
3.4 Primary Interviews
4. Market Dynamics Impact Analysis
4.1 Market Driving Factors Analysis
4.1.1 Drivers
4.1.2 Restraints
4.1.3 Opportunities
4.1.4 Challenges
4.2 PESTLE Analysis
4.3 Porter’s Five Forces Model
5. Statistical Insights and Trends Reporting
5.1 Sterility Testing Demand by Drug Type (2023)
5.2 Technological Trends in Sterility Testing (2023-2032)
5.3 Regulatory Compliance and Standards (2023-2032)
5.4 Outsourcing Trends in Sterility Testing (2023)
6. Competitive Landscape
6.1 List of Major Companies, By Region
6.2 Market Share Analysis, By Region
6.3 Product Benchmarking
6.3.1 Product specifications and features
6.3.2 Pricing
6.4 Strategic Initiatives
6.4.1 Marketing and promotional activities
6.4.2 Distribution and supply chain strategies
6.4.3 Expansion plans and new product launches
6.4.4 Strategic partnerships and collaborations
6.5 Technological Advancements
6.6 Market Positioning and Branding
7. Pharmaceutical Sterility Testing Market Segmentation, by Type
7.1 Chapter Overview
7.2 In-House
7.2.1 In-House Market Trends Analysis (2020-2032)
7.2.2 In-House Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3 Outsourcing
7.3.1 Outsourcing Market Trends Analysis (2020-2032)
7.3.2 Outsourcing Market Size Estimates and Forecasts to 2032 (USD Billion)
8. Pharmaceutical Sterility Testing Market Segmentation, by Product Type
8.1 Chapter Overview
8.2 Kits & Reagents
8.2.1 Kits & Reagents Market Trends Analysis (2020-2032)
8.2.2 Kits & Reagents Market Size Estimates and Forecasts to 2032 (USD Billion)
8.3 Instruments
8.3.1 Instruments Market Trends Analysis (2020-2032)
8.3.2 Instruments Market Size Estimates and Forecasts to 2032 (USD Billion)
8.4 Services
8.4.1 Services Market Trends Analysis (2020-2032)
8.4.2 Services Market Size Estimates and Forecasts to 2032 (USD Billion)
9. Pharmaceutical Sterility Testing Market Segmentation, by End Use
9.1 Chapter Overview
9.2 Compounding Pharmacies
9.2.1 Compounding Pharmacies Market Trends Analysis (2020-2032)
9.2.2 Compounding Pharmacies Market Size Estimates and Forecasts to 2032 (USD Billion)
9.3 Medical Device Companies
9.3.1 Medical Device Companies Market Trends Analysis (2020-2032)
9.3.2 Medical Device Companies Market Size Estimates and Forecasts to 2032 (USD Billion)
9.4 Pharmaceutical Companies
9.4.1 Pharmaceutical Companies Market Trends Analysis (2020-2032)
9.4.2 Pharmaceutical Companies Market Size Estimates and Forecasts to 2032 (USD Billion)
10. Regional Analysis
10.1 Chapter Overview
10.2 North America
10.2.1 Trends Analysis
10.2.2 North America Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.2.3 North America Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.2.4 North America Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.2.5 North America Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.2.6 USA
10.2.6.1 USA Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.2.6.2 USA Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.2.6.3 USA Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.2.7 Canada
10.2.7.1 Canada Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.2.7.2 Canada Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.2.7.3 Canada Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.2.8 Mexico
10.2.8.1 Mexico Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.2.8.2 Mexico Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.2.8.3 Mexico Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.3 Europe
10.3.1 Eastern Europe
10.3.1.1 Trends Analysis
10.3.1.2 Eastern Europe Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.3.1.3 Eastern Europe Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.1.4 Eastern Europe Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.3.1.5 Eastern Europe Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.3.1.6 Poland
10.3.1.6.1 Poland Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.1.6.2 Poland Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.3.1.6.3 Poland Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.3.1.7 Romania
10.3.1.7.1 Romania Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.1.7.2 Romania Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.3.1.7.3 Romania Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.3.1.8 Hungary
10.3.1.8.1 Hungary Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.1.8.2 Hungary Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.3.1.8.3 Hungary Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.3.1.9 Turkey
10.3.1.9.1 Turkey Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.1.9.2 Turkey Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.3.1.9.3 Turkey Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.3.1.10 Rest of Eastern Europe
10.3.1.10.1 Rest of Eastern Europe Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.1.10.2 Rest of Eastern Europe Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.3.1.10.3 Rest of Eastern Europe Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.3.2 Western Europe
10.3.2.1 Trends Analysis
10.3.2.2 Western Europe Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.3.2.3 Western Europe Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.2.4 Western Europe Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.3.2.5 Western Europe Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.3.2.6 Germany
10.3.2.6.1 Germany Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.2.6.2 Germany Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.3.2.6.3 Germany Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.3.2.7 France
10.3.2.7.1 France Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.2.7.2 France Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.3.2.7.3 France Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.3.2.8 UK
10.3.2.8.1 UK Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.2.8.2 UK Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.3.2.8.3 UK Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.3.2.9 Italy
10.3.2.9.1 Italy Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.2.9.2 Italy Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.3.2.9.3 Italy Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.3.2.10 Spain
10.3.2.10.1 Spain Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.2.10.2 Spain Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.3.2.10.3 Spain Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.3.2.11 Netherlands
10.3.2.11.1 Netherlands Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.2.11.2 Netherlands Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.3.2.11.3 Netherlands Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.3.2.12 Switzerland
10.3.2.12.1 Switzerland Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.2.12.2 Switzerland Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.3.2.12.3 Switzerland Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.3.2.13 Austria
10.3.2.13.1 Austria Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.2.13.2 Austria Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.3.2.13.3 Austria Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.3.2.14 Rest of Western Europe
10.3.2.14.1 Rest of Western Europe Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.2.14.2 Rest of Western Europe Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.3.2.14.3 Rest of Western Europe Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.4 Asia Pacific
10.4.1 Trends Analysis
10.4.2 Asia Pacific Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.4.3 Asia Pacific Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.4.4 Asia Pacific Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.4.5 Asia Pacific Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.4.6 China
10.4.6.1 China Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.4.6.2 China Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.4.6.3 China Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.4.7 India
10.4.7.1 India Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.4.7.2 India Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.4.7.3 India Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.4.8 Japan
10.4.8.1 Japan Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.4.8.2 Japan Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.4.8.3 Japan Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.4.9 South Korea
10.4.9.1 South Korea Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.4.9.2 South Korea Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.4.9.3 South Korea Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.4.10 Vietnam
10.4.10.1 Vietnam Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.4.10.2 Vietnam Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.4.10.3 Vietnam Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.4.11 Singapore
10.4.11.1 Singapore Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.4.11.2 Singapore Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.4.11.3 Singapore Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.4.12 Australia
10.4.12.1 Australia Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.4.12.2 Australia Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.4.12.3 Australia Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.4.13 Rest of Asia Pacific
10.4.13.1 Rest of Asia Pacific Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.4.13.2 Rest of Asia Pacific Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.4.13.3 Rest of Asia Pacific Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.5 Middle East and Africa
10.5.1 Middle East
10.5.1.1 Trends Analysis
10.5.1.2 Middle East Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.5.1.3 Middle East Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.5.1.4 Middle East Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.5.1.5 Middle East Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.5.1.6 UAE
10.5.1.6.1 UAE Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.5.1.6.2 UAE Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.5.1.6.3 UAE Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.5.1.7 Egypt
10.5.1.7.1 Egypt Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.5.1.7.2 Egypt Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.5.1.7.3 Egypt Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.5.1.8 Saudi Arabia
10.5.1.8.1 Saudi Arabia Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.5.1.8.2 Saudi Arabia Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.5.1.8.3 Saudi Arabia Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.5.1.9 Qatar
10.5.1.9.1 Qatar Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.5.1.9.2 Qatar Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.5.1.9.3 Qatar Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.5.1.10 Rest of Middle East
10.5.1.10.1 Rest of Middle East Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.5.1.10.2 Rest of Middle East Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.5.1.10.3 Rest of Middle East Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.5.2 Africa
10.5.2.1 Trends Analysis
10.5.2.2 Africa Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.5.2.3 Africa Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.5.2.4 Africa Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.5.2.5 Africa Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.5.2.6 South Africa
10.5.2.6.1 South Africa Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.5.2.6.2 South Africa Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.5.2.6.3 South Africa Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.5.2.7 Nigeria
10.5.2.7.1 Nigeria Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.5.2.7.2 Nigeria Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.5.2.7.3 Nigeria Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.5.2.8 Rest of Africa
10.5.2.8.1 Rest of Africa Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.5.2.8.2 Rest of Africa Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.5.2.8.3 Rest of Africa Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.6 Latin America
10.6.1 Trends Analysis
10.6.2 Latin America Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.6.3 Latin America Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.6.4 Latin America Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.6.5 Latin America Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.6.6 Brazil
10.6.6.1 Brazil Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.6.6.2 Brazil Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.6.6.3 Brazil Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.6.7 Argentina
10.6.7.1 Argentina Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.6.7.2 Argentina Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.6.7.3 Argentina Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.6.8 Colombia
10.6.8.1 Colombia Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.6.8.2 Colombia Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.6.8.3 Colombia Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.6.9 Rest of Latin America
10.6.9.1 Rest of Latin America Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.6.9.2 Rest of Latin America Pharmaceutical Sterility Testing Market Estimates and Forecasts, by Product Type (2020-2032) (USD Billion)
10.6.9.3 Rest of Latin America Pharmaceutical Sterility Testing Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
11. Company Profiles
11.1 Pacific Biolabs
11.1.1 Company Overview
11.1.2 Financial
11.1.3 Products/ Services Offered
11.1.4 SWOT Analysis
11.2 Steris Plc
11.2.1 Company Overview
11.2.2 Financial
11.2.3 Products/ Services Offered
11.2.4 SWOT Analysis
11.3 Boston Analytical
11.3.1 Company Overview
11.3.2 Financial
11.3.3 Products/ Services Offered
11.3.4 SWOT Analysis
11.4 Sotera Health Company
11.4.1 Company Overview
11.4.2 Financial
11.4.3 Products/ Services Offered
11.4.4 SWOT Analysis
11.5 Sartorius Ag
11.5.1 Company Overview
11.5.2 Financial
11.5.3 Products/ Services Offered
11.5.4 SWOT Analysis
11.6 Labcorp
11.6.1 Company Overview
11.6.2 Financial
11.6.3 Products/ Services Offered
11.6.4 SWOT Analysis
11.7 Pace Analytical
11.7.1 Company Overview
11.7.2 Financial
11.7.3 Products/ Services Offered
11.7.4 SWOT Analysis
11.8 Charles River Laboratories
11.8.1 Company Overview
11.8.2 Financial
11.8.3 Products/ Services Offered
11.8.4 SWOT Analysis
11.9 Thermo Fisher Scientific, Inc.
11.9.1 Company Overview
11.9.2 Financial
11.9.3 Products/ Services Offered
11.9.4 SWOT Analysis
11.10 Rapid Micro Biosystems, Inc.
11.10.1 Company Overview
11.10.2 Financial
11.10.3 Products/ Services Offered
11.10.4 SWOT Analysis
12. Use Cases and Best Practices
13. Conclusion
An accurate research report requires proper strategizing as well as implementation. There are multiple factors involved in the completion of good and accurate research report and selecting the best methodology to compete the research is the toughest part. Since the research reports we provide play a crucial role in any company’s decision-making process, therefore we at SNS Insider always believe that we should choose the best method which gives us results closer to reality. This allows us to reach at a stage wherein we can provide our clients best and accurate investment to output ratio.
Each report that we prepare takes a timeframe of 350-400 business hours for production. Starting from the selection of titles through a couple of in-depth brain storming session to the final QC process before uploading our titles on our website we dedicate around 350 working hours. The titles are selected based on their current market cap and the foreseen CAGR and growth.
The 5 steps process:
Step 1: Secondary Research:
Secondary Research or Desk Research is as the name suggests is a research process wherein, we collect data through the readily available information. In this process we use various paid and unpaid databases which our team has access to and gather data through the same. This includes examining of listed companies’ annual reports, Journals, SEC filling etc. Apart from this our team has access to various associations across the globe across different industries. Lastly, we have exchange relationships with various university as well as individual libraries.

Step 2: Primary Research
When we talk about primary research, it is a type of study in which the researchers collect relevant data samples directly, rather than relying on previously collected data. This type of research is focused on gaining content specific facts that can be sued to solve specific problems. Since the collected data is fresh and first hand therefore it makes the study more accurate and genuine.
We at SNS Insider have divided Primary Research into 2 parts.
Part 1 wherein we interview the KOLs of major players as well as the upcoming ones across various geographic regions. This allows us to have their view over the market scenario and acts as an important tool to come closer to the accurate market numbers. As many as 45 paid and unpaid primary interviews are taken from both the demand and supply side of the industry to make sure we land at an accurate judgement and analysis of the market.
This step involves the triangulation of data wherein our team analyses the interview transcripts, online survey responses and observation of on filed participants. The below mentioned chart should give a better understanding of the part 1 of the primary interview.

Part 2: In this part of primary research the data collected via secondary research and the part 1 of the primary research is validated with the interviews from individual consultants and subject matter experts.
Consultants are those set of people who have at least 12 years of experience and expertise within the industry whereas Subject Matter Experts are those with at least 15 years of experience behind their back within the same space. The data with the help of two main processes i.e., FGDs (Focused Group Discussions) and IDs (Individual Discussions). This gives us a 3rd party nonbiased primary view of the market scenario making it a more dependable one while collation of the data pointers.
Step 3: Data Bank Validation
Once all the information is collected via primary and secondary sources, we run that information for data validation. At our intelligence centre our research heads track a lot of information related to the market which includes the quarterly reports, the daily stock prices, and other relevant information. Our data bank server gets updated every fortnight and that is how the information which we collected using our primary and secondary information is revalidated in real time.

Step 4: QA/QC Process
After all the data collection and validation our team does a final level of quality check and quality assurance to get rid of any unwanted or undesired mistakes. This might include but not limited to getting rid of the any typos, duplication of numbers or missing of any important information. The people involved in this process include technical content writers, research heads and graphics people. Once this process is completed the title gets uploader on our platform for our clients to read it.
Step 5: Final QC/QA Process:
This is the last process and comes when the client has ordered the study. In this process a final QA/QC is done before the study is emailed to the client. Since we believe in giving our clients a good experience of our research studies, therefore, to make sure that we do not lack at our end in any way humanly possible we do a final round of quality check and then dispatch the study to the client.
Key Segments:
By Type
In-House
Outsourcing
By Product Type
Kits & Reagents
Instruments
Services
By End-use
Compounding Pharmacies
Medical Device Companies
Pharmaceutical Companies
Request for Segment Customization as per your Business Requirement: Segment Customization Request
Regional Coverage:
North America
US
Canada
Mexico
Europe
Eastern Europe
Poland
Romania
Hungary
Turkey
Rest of Eastern Europe
Western Europe
Germany
France
UK
Italy
Spain
Netherlands
Switzerland
Austria
Rest of Western Europe
Asia Pacific
China
India
Japan
South Korea
Vietnam
Singapore
Australia
Rest of Asia Pacific
Middle East & Africa
Middle East
UAE
Egypt
Saudi Arabia
Qatar
Rest of the Middle East
Africa
Nigeria
South Africa
Rest of Africa
Latin America
Brazil
Argentina
Colombia
Rest of Latin America
Request for Country Level Research Report: Country Level Customization Request
Available Customization
With the given market data, SNS Insider offers customization as per the company’s specific needs. The following customization options are available for the report:
Product Analysis
Criss-Cross segment analysis (e.g. Product X Application)
Product Matrix which gives a detailed comparison of the product portfolio of each company
Geographic Analysis
Additional countries in any of the regions
Company Information
Detailed analysis and profiling of additional market players (Up to five)
The Contrast Media Injectors Market size was valued at USD 1.34 Billion in 2023 & will reach USD 2.57 Bn by 2032 with a growing CAGR of 7.62% by 2024-2032.
Cancer Registry Software Market Size was valued at USD 76.6 Million in 2023 and is expected to reach USD 211.1 Million by 2032, growing at a CAGR of 11.9% over the forecast period 2024-2032.
The Inhalable Drug Delivery Systems Market was valued at USD 2.37 billion in 2023, expected to reach USD 3.81 billion by 2032 growing at a CAGR of 4.95%.
Transport Chairs Market was valued at USD 1.2 billion in 2023 and is expected to reach USD 2.26 billion by 2032, growing at a CAGR of 7.28% over the forecast period of 2024-2032.
The Medical Device Testing Services Market was valued at USD 8.95 billion in 2023 and is expected to reach USD 20.30 billion by 2032, growing at a CAGR of 9.55% over the forecast period of 2024-2032.
The global 3D Cell Culture Market, valued at USD 1.4 billion in 2023 and projected to reach USD 4.0 billion by 2032, growing at a CAGR of 12.4% by 2032.
Hi! Click one of our member below to chat on Phone