To Get More Information on NICU Catheters Market - Request Sample Report
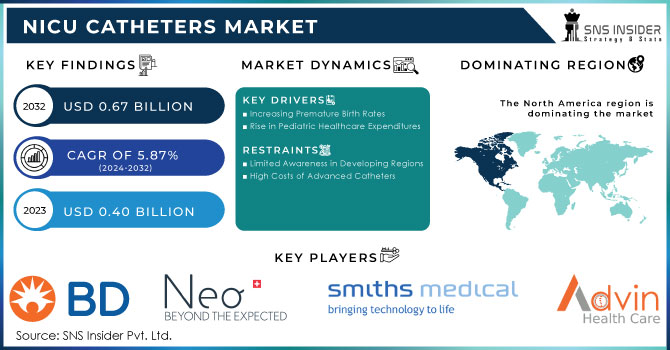
NICU Catheters Market size was valued at USD 400 Million in 2023 and is expected to reach USD 670 Million by 2032, growing at a CAGR of 5.87% from 2024-2032.
Recent trends of increasing preterm births and complex medical conditions among neonates have escalated the demand for specialized catheter solutions in neonatal intensive care. It is reported that in 2020, an estimated 13.4 million babies were born preterm while preterm birth rates are between 4-16% globally and therefore there would be immense future demand in the coming years. This comes with a serious need for these medical devices to be safe and effective for giving drugs, nutrition, and fluids to fragile newborns, given the fact that in 2022, 2.3 million children died within the first 20 days of life.
Several factors drive the growth of the NICU catheters market, such as increased awareness regarding health issues related to neonates and enhancements in healthcare infrastructure. The increase in NICUs, especially in developing regions, showcases an increased demand for high-quality catheter products. A huge number of NICU admissions are still significant; 11.9% of all births and 4.1% of high-acuity births are admitted into specialized neonatal care, which further increases the need for sophisticated catheter solutions.
Advanced technologies like ECMO, EPIV catheters, Microseldinger Technique (MST), ultrasonography-assisted catheter placement, and inhaled nitric oxide therapy for PPHN make it possible for manufacturers to create solutions specifically targeted at vulnerable neonates. Access to healthcare is increasing in other developing regions, and that is going to spur such advanced innovations to become even stronger. Collaboration among the manufacturers, healthcare service providers, and regulatory agencies will ensure that the clinical needs are met and these innovations lead to good neonatal health outcomes and other growth opportunities in the NICU catheter market.
DRIVERS
Increasing Premature Birth Rates
In the last few years, the rate of preterm births has risen sharply and has gained traction in catheters used in the NICU, as these tiny babies are exposed to critical medical treatment. In the 2023 Report Card, 380,548 babies are reported to have been born at less than 37 weeks of gestation in the United States, thus highlighting the extremely critical demand for proper neonatal care. Maternal medical conditions, multiple pregnancies, socioeconomic factors, and an older maternal age population are also among the other factors contributing to a high rate of preterm delivery. This trend is bringing enormous growth opportunities to the market, and hence healthcare systems will be investing in NICU facilities and innovative catheter designs specifically tailored for neonatal care. Added to this will be further integration of products with the healthcare providers and education purposes from direct manufacturers, which will again boost demand in emerging markets where awareness about neonatal health is on the rise.
Rise in Pediatric Healthcare Expenditures
Pediatric healthcare expenditure is estimated to have reached about USD 13.02 billion in 2023 globally. Such an investment imposes a huge expenditure in the NICU catheters market as healthcare systems look to better neonatal care. Such investments are immense and have a measure beyond the advancement of patient outcomes alone; instead, they promote new-age technology for vulnerable newborns. Additional investments in pediatric care would further be instrumental in building more NICUs, which increases the need for better catheter products. Overall, the investment in terms of finance contributes to progressing market growth toward improved neonatal care.
RESTRAINTS
Limited Awareness in Developing Regions
An important challenge for the market of NICU catheters lies in low awareness levels in developing regions, particularly in low and middle-income countries. In this region, because of a lack of awareness about the benefits of such specific catheters in the practicing and healthcare arenas, the therapeutic applications of these critical devices are less utilized. A limited awareness level regarding the availability of such catheters might discourage the adoption of various advanced neonatal care practices by practitioners and thus impact patient outcomes. On the other hand, the reason for inappropriate training and resources is a bigger issue, therefore, calling for education and awareness drive to be improved to enhance this current condition. Important to bridge this gap by increasing the usage of specialized catheters in delivering adequate health care to vulnerable newborn babies.
High Costs of Advanced Catheters
The limiting factor of this market is mainly because of the high costs associated with advanced NICU catheters. It is very expensive, and thus not found easily in resource-constrained healthcare settings. Most such devices are very costly to produce and procure and hence rarely seen in the markets. The average cost of care within the NICU varies at USD16,800 for each infant with a NICU-associated bloodstream infection. This barrier may prevent hospitals from investing in catheter technologies of necessity, which may have a destructive impact on quality neonatal care service delivery. It is important to conquer such cost-related barriers if superior access to advanced catheter solutions is to be experienced in all healthcare environments.
BY PRODUCT
Peripherally Inserted Central Catheters dominated the NICU catheters market in the year 2023, and had a revenue share that closely approximates about 52%, primarily because they are relatively versatile and their insertion procedure is rather easy. Their design makes them suitable for long-term venous access while offering neonates prolonged treatment without much risk associated with more invasive procedures.
On the other hand, CVCs are expected to grow at a higher rate with a CAGR of about 6.30% between the period of 2024 to 2032, due to the rising prevalence of acute medical conditions among infants who are directly observed and treated. After all, CVCs provide easy access for the administration of treatments that cannot be administered through peripheral routes: which is a key part of the treatment for neonates. Accordingly, this new trend of focusing on the betterment of outcomes in vulnerable newborns will make PICCs and CVCs cornerstones in developing better treatment options.
BY END USER
Hospitals are the largest contributors in the NICU catheters market, with a share of around 63% of revenue, as they have more resources and specialized human resources in neonatal care. These medical facilities are equipped with state-of-the-art technology and infrastructure, which facilitates the care of high-risk infants who need intensified treatment and monitoring.
However, specialty clinics are projected to hold the highest CAGR at around 6.54% from 2024-2032. Specialty clinics will experience a rise due to the growing availability of outpatient care and dedicated services for infants with specific medical requirements. Since families search for more customized therapies, specialty clinics are integral to the developing neonatal care network and consequently surge the NICU catheters market.
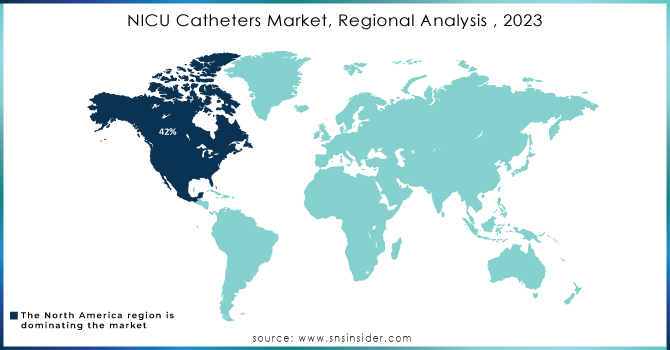
By 2023, North America was projected to account for nearly 42% of the NICU catheters market in terms of revenue share. This strong position is primarily due to its sophisticated healthcare infrastructure, significant investments in pediatric care, and a dedicated focus on research and innovation in neonatology, along with the utilization of advanced medical technology. The high number of specialized hospitals and clinics further boosts the demand for NICU catheters. According to a 2023 study, 1,424 hospitals in the U.S. had operational NICUs, totaling 35,601 NICU beds. This includes 5,592 Level II beds, 20,631 Level III beds, and 9,378 Level IV beds, highlighting the extensive capacity that supports the region's leadership in the NICU catheter market.
The Asia-Pacific region is expected to be one of the most dominant markets in terms of growth, where NICU catheters can achieve a CAGR of around 6.59% during 2024 and 2032. In 2022, premature birth percentage across Asia was estimated to be 8.1%, whereas preterm births were 4.1% for the period 2022-2023 in India, occurring before 37 weeks of gestation. There is increased healthcare spending, high awareness regarding neonatal care, and population demand as major growth indicators. With these growth indicators, the market of high-end NICU catheters is expected to dominate the regional market as healthcare technologies continue to gain momentum in this region. These above factors indicate that the market of global NICU catheters is highly dynamic and ever-changing.
Do You Need any Customization Research on NICU Catheters Market - Inquire Now
IN 2023, ICU Medical has just announced that its Plum Duo infusion pump, featuring LifeShield infusion safety software, has received FDA 510(k) clearance. The sophisticated pump and software will begin shipping to U.S. customers in early 2024.
In 2023, several NHS Trusts across the UK began using a new umbilical catheter that incorporates the AgION antimicrobial system. It has already proven to be effective in limiting preterm NICU patients' potentially life-threatening, or at least, potentially severe CRBSIs.
Becton, Dickinson and Company (BD Nexiva Closed IV Catheter System, BD Insyte Autoguard IV Catheter)
Footprint Medical Incorporated (Footprint Medical Catheter Securement Device, Footprint Medical IV Catheter Stabilization Device)
Vigmed AB (Vigmed Safety Catheter, Vigmed VigiFlow Catheter)
NeoMedical Inc. (NeoMedical NeoCat Catheter, NeoMedical NeoAccess Catheter)
Smiths Medical (Portex Pediatric Catheters, Medex Catheter Products)
Advin Health Care (Advin PICC Catheters, Advin Peripheral Catheters)
ICU Medical Inc. (ICU Medical Dual-Lumen Catheter, ICU Medical CLAVE Connector)
Marian Medical Inc. (Marian Medical PICC Catheters, Marian Medical Pediatric IV Catheters)
Bactiguard AB (Bactiguard Infection Protection Catheters, Bactiguard Pediatric Catheters)
Cardinal Health Inc. (Cardinal Health PICC Catheters, Cardinal Health Peripheral IV Catheters)
Abbott (U.S) (Abbott Freestyle Libre Sensors, Abbott PICC Catheters)
Boston Scientific Corporation (U.S) (Boston Scientific Rhythmia Catheter, Boston Scientific EP Catheter)
Medtronic (Ireland) (Medtronic Swan-Ganz Catheter, Medtronic TLD Catheter)
Cook (U.S) (Cook Pediatric PICC Catheter, Cook Introducer Sheath)
Johnson & Johnson Private Limited (U.S) (Ethicon Catheters, Mentor Catheters)
Smith & Nephew plc (Germany) (Smith & Nephew IV Catheters, Smith & Nephew Securement Devices)
Cardinal Health (U.S) (Cardinal Health PICC Catheters, Cardinal Health Peripheral IV Catheters)
Terumo Corporation (Japan) (Terumo Surflo IV Catheters, Terumo PICC Catheters)
Conavi Medical (U.S) (Conavi Medical Imaging Catheters, Conavi Medical Catheter Systems)
Edwards Lifesciences Corporation (U.S) (Edwards Lifesciences Central Venous Catheters, Edwards Lifesciences PICC Catheters)
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | USD 400 Million |
| Market Size by 2032 | USD 670 Million |
| CAGR | CAGR of 5.87% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Product(Peripherally Inserted Central Catheters (PICCs), Central Venous Catheters (CVCs), Umbilical Venous Catheters (UVCs), Others) • By End User(Hospitals , Ambulatory Surgery Center,Specialty Clinics,Others ) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | CONMED Corporation, Orthofix US LLC, Wright Medical Group N.V., NuVasive, Inc, Corin Group, Enovis, OsteoMed, Invibio Ltd., gpcmedical.com, Medtronic, Integra LifeSciences, B. Braun SE, Stryker, Zimmer Biomet, Smith+Nephew, Advanced Orthopaedic Solutions, Acumed LLC, Electramed Ltd, Implantate AG, Bioretec Ltd, citieffe s.r.l |
| Key Drivers | •The rise in preterm births is driving demand for specialized NICU catheters and boosting investments in neonatal care. • Global pediatric healthcare spending is boosting investments in NICU catheters and technology, advancing neonatal care and market growth.. |
| RESTRAINTS | •Limited awareness of NICU catheters in developing regions hinders their use and underscores the need for better education. •High costs of advanced NICU catheters limit their availability in resource-constrained healthcare settings, hindering quality neonatal care. |
Ans: NICU Catheters Market was valued at USD 0.40 billion in 2023 and is expected to reach USD 0.67 billion by 2032, growing at a CAGR of 5.87% from 2024-2032.
Ans: North America holds the largest share of the NICU catheter market, approximately 42.36%.
Ans: Key growth drivers include rising rates of premature births, increased pediatric healthcare expenditures, and advancements in neonatal care technologies.
Ans: The primary types are Peripherally Inserted Central Catheters (PICCs) and Central Venous Catheters (CVCs).
Table of Content
1. Introduction
1.1 Market Definition
1.2 Scope (Inclusion and Exclusions)
1.3 Research Assumptions
2. Executive Summary
2.1 Market Overview
2.2 Regional Synopsis
2.3 Competitive Summary
3. Research Methodology
3.1 Top-Down Approach
3.2 Bottom-up Approach
3.3. Data Validation
3.4 Primary Interviews
4. Market Dynamics Impact Analysis
4.1 Market Driving Factors Analysis
4.1.2 Drivers
4.1.2 Restraints
4.1.3 Opportunities
4.1.4 Challenges
4.2 PESTLE Analysis
4.3 Porter’s Five Forces Model
5. Statistical Insights and Trends Reporting
5.1 Incidence and Prevalence (2023)
5.2 Prescription Trends, (2023), by Region
5.3 Device Volume, by Region (2020-2032)
5.4 Healthcare Spending, by region, (Government, Commercial, Private, Out-of-Pocket), 2023
6. Competitive Landscape
6.1 List of Major Companies, By Region
6.2 Market Share Analysis, By Region
6.3 Product Benchmarking
6.3.1 Product specifications and features
6.3.2 Pricing
6.4 Strategic Initiatives
6.4.1 Marketing and promotional activities
6.4.2 Distribution and supply chain strategies
6.4.3 Expansion plans and new product launches
6.4.4 Strategic partnerships and collaborations
6.5 Technological Advancements
6.6 Market Positioning and Branding
7. NICU Catheters Market Segmentation, by Product
7.1 Chapter Overview
7.2 Peripherally Inserted Central Catheters (PICCs)
7.2.1 Peripherally Inserted Central Catheters (PICCs) Market Trends Analysis (2020-2032)
7.2.2 Peripherally Inserted Central Catheters (PICCs) Market Size Estimates and Forecasts to 2032 (USD Million)
7.3 Central Venous Catheters (CVCs)
7.3.1 Central Venous Catheters (CVCs) Market Trends Analysis (2020-2032)
7.3.2 Central Venous Catheters (CVCs) Market Size Estimates and Forecasts to 2032 (USD Million)
7.4 Umbilical Venous Catheters (UVCs)
7.4.1 Umbilical Venous Catheters (UVCs) Market Trends Analysis (2020-2032)
7.4.2 Umbilical Venous Catheters (UVCs) Market Size Estimates and Forecasts to 2032 (USD Million)
7.5 Others
7.5.1 Others Market Trends Analysis (2020-2032)
7.5.2 Others Market Size Estimates and Forecasts to 2032 (USD Million)
8. NICU Catheters Market Segmentation, by End User
8.1 Chapter Overview
8.2 Hospitals
8.2.1 Hospitals Market Trends Analysis (2020-2032)
8.2.2 Hospitals Market Size Estimates and Forecasts to 2032 (USD Million)
8.3 Ambulatory Surgical Centers
8.3.1 Ambulatory Surgical Centers Market Trends Analysis (2020-2032)
8.3.2 Ambulatory Surgical Centers Market Size Estimates and Forecasts to 2032 (USD Million)
8.4 Specialty Clinics
8.4.1 Specialty Clinics Market Trends Analysis (2020-2032)
8.4.2 Specialty Clinics Market Size Estimates and Forecasts to 2032 (USD Million)
8.5 Others
8.5.1 Others Market Trends Analysis (2020-2032)
8.5.2 Others Market Size Estimates and Forecasts to 2032 (USD Million)
9. Regional Analysis
9.1 Chapter Overview
9.2 North America
9.2.1 Trends Analysis
9.2.2 North America NICU Catheters Market Estimates and Forecasts, by Country (2020-2032) (USD Million)
9.2.3 North America NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.2.4 North America NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.2.5 USA
9.2.5.1 USA NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.2.5.2 USA NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.2.6 Canada
9.2.6.1 Canada NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.2.6.2 Canada NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.2.7 Mexico
9.2.7.1 Mexico NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.2.7.2 Mexico NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.3 Europe
9.3.1 Eastern Europe
9.3.1.1 Trends Analysis
9.3.1.2 Eastern Europe NICU Catheters Market Estimates and Forecasts, by Country (2020-2032) (USD Million)
9.3.1.3 Eastern Europe NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.3.1.4 Eastern Europe NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.3.1.5 Poland
9.3.1.5.1 Poland NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.3.1.5.2 Poland NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.3.1.6 Romania
9.3.1.6.1 Romania NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.3.1.6.2 Romania NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.3.1.7 Hungary
9.3.1.7.1 Hungary NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.3.1.7.2 Hungary NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.3.1.8 Turkey
9.3.1.8.1 Turkey NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.3.1.8.2 Turkey NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.3.1.9 Rest of Eastern Europe
9.3.1.9.1 Rest of Eastern Europe NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.3.1.9.2 Rest of Eastern Europe NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.3.2 Western Europe
9.3.2.1 Trends Analysis
9.3.2.2 Western Europe NICU Catheters Market Estimates and Forecasts, by Country (2020-2032) (USD Million)
9.3.2.3 Western Europe NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.3.2.4 Western Europe NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.3.2.5 Germany
9.3.2.5.1 Germany NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.3.2.5.2 Germany NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.3.2.6 France
9.3.2.6.1 France NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.3.2.6.2 France NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.3.2.7 UK
9.3.2.7.1 UK NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.3.2.7.2 UK NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.3.2.8 Italy
9.3.2.8.1 Italy NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.3.2.8.2 Italy NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.3.2.9 Spain
9.3.2.9.1 Spain NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.3.2.9.2 Spain NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.3.2.10 Netherlands
9.3.2.10.1 Netherlands NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.3.2.10.2 Netherlands NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.3.2.11 Switzerland
9.3.2.11.1 Switzerland NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.3.2.11.2 Switzerland NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.3.2.12 Austria
9.3.2.12.1 Austria NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.3.2.12.2 Austria NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.3.2.13 Rest of Western Europe
9.3.2.13.1 Rest of Western Europe NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.3.2.13.2 Rest of Western Europe NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.4 Asia Pacific
9.4.1 Trends Analysis
9.4.2 Asia Pacific NICU Catheters Market Estimates and Forecasts, by Country (2020-2032) (USD Million)
9.4.3 Asia Pacific NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.4.4 Asia Pacific NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.4.5 China
9.4.5.1 China NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.4.5.2 China NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.4.6 India
9.4.5.1 India NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.4.5.2 India NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.4.5 Japan
9.4.5.1 Japan NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.4.5.2 Japan NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.4.6 South Korea
9.4.6.1 South Korea NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.4.6.2 South Korea NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.4.7 Vietnam
9.4.7.1 Vietnam NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.2.7.2 Vietnam NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.4.8 Singapore
9.4.8.1 Singapore NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.4.8.2 Singapore NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.4.9 Australia
9.4.9.1 Australia NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.4.9.2 Australia NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.4.10 Rest of Asia Pacific
9.4.10.1 Rest of Asia Pacific NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.4.10.2 Rest of Asia Pacific NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.5 Middle East and Africa
9.5.1 Middle East
9.5.1.1 Trends Analysis
9.5.1.2 Middle East NICU Catheters Market Estimates and Forecasts, by Country (2020-2032) (USD Million)
9.5.1.3 Middle East NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.5.1.4 Middle East NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.5.1.5 UAE
9.5.1.5.1 UAE NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.5.1.5.2 UAE NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.5.1.6 Egypt
9.5.1.6.1 Egypt NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.5.1.6.2 Egypt NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.5.1.7 Saudi Arabia
9.5.1.7.1 Saudi Arabia NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.5.1.7.2 Saudi Arabia NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.5.1.8 Qatar
9.5.1.8.1 Qatar NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.5.1.8.2 Qatar NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.5.1.9 Rest of Middle East
9.5.1.9.1 Rest of Middle East NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.5.1.9.2 Rest of Middle East NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.5.2 Africa
9.5.2.1 Trends Analysis
9.5.2.2 Africa NICU Catheters Market Estimates and Forecasts, by Country (2020-2032) (USD Million)
9.5.2.3 Africa NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.5.2.4 Africa NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.5.2.5 South Africa
9.5.2.5.1 South Africa NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.5.2.5.2 South Africa NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.5.2.6 Nigeria
9.5.2.6.1 Nigeria NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.5.2.6.2 Nigeria NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.5.2.7 Rest of Africa
9.5.2.7.1 Rest of Africa NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.5.2.7.2 Rest of Africa NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.6 Latin America
9.6.1 Trends Analysis
9.6.2 Latin America NICU Catheters Market Estimates and Forecasts, by Country (2020-2032) (USD Million)
9.6.3 Latin America NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.6.4 Latin America NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.6.5 Brazil
9.6.5.1 Brazil NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.6.5.2 Brazil NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.6.6 Argentina
9.6.6.1 Argentina NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.6.6.2 Argentina NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.6.7 Colombia
9.6.7.1 Colombia NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.6.7.2 Colombia NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
9.6.8 Rest of Latin America
9.6.8.1 Rest of Latin America NICU Catheters Market Estimates and Forecasts, by Product (2020-2032) (USD Million)
9.6.8.2 Rest of Latin America NICU Catheters Market Estimates and Forecasts, by End User (2020-2032) (USD Million)
10. Company Profiles
10.1 Becton, Dickinson and Company
10.1.1 Company Overview
10.1.2 Financial
10.1.3 Products/ Services Offered
110.1.4 SWOT Analysis
10.2 Vigmed AB
10.2.1 Company Overview
10.2.2 Financial
10.2.3 Products/ Services Offered
10.2.4 SWOT Analysis
10.3 NeoMedical Inc.
10.3.1 Company Overview
10.3.2 Financial
10.3.3 Products/ Services Offered
10.3.4 SWOT Analysis
10.4 Advin Health Care
10.4.1 Company Overview
10.4.2 Financial
10.4.3 Products/ Services Offered
10.4.4 SWOT Analysis
10.5 ICU Medical Inc
10.5.1 Company Overview
10.5.2 Financial
10.5.3 Products/ Services Offered
10.5.4 SWOT Analysis
10.6 Bactiguard AB
10.6.1 Company Overview
10.6.2 Financial
10.6.3 Products/ Services Offered
10.6.4 SWOT Analysis
10.7 Abbott
10.7.1 Company Overview
10.7.2 Financial
10.7.3 Products/ Services Offered
10.7.4 SWOT Analysis
10.8 Johnson & Johnson Private Limited
10.8.1 Company Overview
10.8.2 Financial
10.8.3 Products/ Services Offered
10.8.4 SWOT Analysis
10.9 Cardinal Health
10.9.1 Company Overview
10.9.2 Financial
10.9.3 Products/ Services Offered
10.9.4 SWOT Analysis
10.10 Edwards Lifesciences Corporation
10.9.1 Company Overview
10.9.2 Financial
10.9.3 Products/ Services Offered
10.9.4 SWOT Analysis
11. Use Cases and Best Practices
12. Conclusion
An accurate research report requires proper strategizing as well as implementation. There are multiple factors involved in the completion of good and accurate research report and selecting the best methodology to compete the research is the toughest part. Since the research reports we provide play a crucial role in any company’s decision-making process, therefore we at SNS Insider always believe that we should choose the best method which gives us results closer to reality. This allows us to reach at a stage wherein we can provide our clients best and accurate investment to output ratio.
Each report that we prepare takes a timeframe of 350-400 business hours for production. Starting from the selection of titles through a couple of in-depth brain storming session to the final QC process before uploading our titles on our website we dedicate around 350 working hours. The titles are selected based on their current market cap and the foreseen CAGR and growth.
The 5 steps process:
Step 1: Secondary Research:
Secondary Research or Desk Research is as the name suggests is a research process wherein, we collect data through the readily available information. In this process we use various paid and unpaid databases which our team has access to and gather data through the same. This includes examining of listed companies’ annual reports, Journals, SEC filling etc. Apart from this our team has access to various associations across the globe across different industries. Lastly, we have exchange relationships with various university as well as individual libraries.

Step 2: Primary Research
When we talk about primary research, it is a type of study in which the researchers collect relevant data samples directly, rather than relying on previously collected data. This type of research is focused on gaining content specific facts that can be sued to solve specific problems. Since the collected data is fresh and first hand therefore it makes the study more accurate and genuine.
We at SNS Insider have divided Primary Research into 2 parts.
Part 1 wherein we interview the KOLs of major players as well as the upcoming ones across various geographic regions. This allows us to have their view over the market scenario and acts as an important tool to come closer to the accurate market numbers. As many as 45 paid and unpaid primary interviews are taken from both the demand and supply side of the industry to make sure we land at an accurate judgement and analysis of the market.
This step involves the triangulation of data wherein our team analyses the interview transcripts, online survey responses and observation of on filed participants. The below mentioned chart should give a better understanding of the part 1 of the primary interview.

Part 2: In this part of primary research the data collected via secondary research and the part 1 of the primary research is validated with the interviews from individual consultants and subject matter experts.
Consultants are those set of people who have at least 12 years of experience and expertise within the industry whereas Subject Matter Experts are those with at least 15 years of experience behind their back within the same space. The data with the help of two main processes i.e., FGDs (Focused Group Discussions) and IDs (Individual Discussions). This gives us a 3rd party nonbiased primary view of the market scenario making it a more dependable one while collation of the data pointers.
Step 3: Data Bank Validation
Once all the information is collected via primary and secondary sources, we run that information for data validation. At our intelligence centre our research heads track a lot of information related to the market which includes the quarterly reports, the daily stock prices, and other relevant information. Our data bank server gets updated every fortnight and that is how the information which we collected using our primary and secondary information is revalidated in real time.

Step 4: QA/QC Process
After all the data collection and validation our team does a final level of quality check and quality assurance to get rid of any unwanted or undesired mistakes. This might include but not limited to getting rid of the any typos, duplication of numbers or missing of any important information. The people involved in this process include technical content writers, research heads and graphics people. Once this process is completed the title gets uploader on our platform for our clients to read it.
Step 5: Final QC/QA Process:
This is the last process and comes when the client has ordered the study. In this process a final QA/QC is done before the study is emailed to the client. Since we believe in giving our clients a good experience of our research studies, therefore, to make sure that we do not lack at our end in any way humanly possible we do a final round of quality check and then dispatch the study to the client.
Key Segments:
By Product
Peripherally Inserted Central Catheters (PICCs)
Central Venous Catheters (CVCs)
Umbilical Venous Catheters (UVCs)
Others
By End User
Hospitals
Specialty Clinics
Ambulatory Surgical Centers
Others
Request for Segment Customization as per your Business Requirement: Segment Customization Request
Regional Coverage:
North America
US
Canada
Mexico
Europe
Eastern Europe
Poland
Romania
Hungary
Turkey
Rest of Eastern Europe
Western Europe
Germany
France
UK
Italy
Spain
Netherlands
Switzerland
Austria
Rest of Western Europe
Asia Pacific
China
India
Japan
South Korea
Vietnam
Singapore
Australia
Rest of Asia Pacific
Middle East & Africa
Middle East
UAE
Egypt
Saudi Arabia
Qatar
Rest of the Middle East
Africa
Nigeria
South Africa
Rest of Africa
Latin America
Brazil
Argentina
Colombia
Rest of Latin America
Request for Country Level Research Report: Country Level Customization Request
Available Customization
With the given market data, SNS Insider offers customization as per the company’s specific needs. The following customization options are available for the report:
Product Analysis
Criss-Cross segment analysis (e.g. Product X Application)
Product Matrix which gives a detailed comparison of the product portfolio of each company
Geographic Analysis
Additional countries in any of the regions
Company Information
Detailed analysis and profiling of additional market players (Up to five)
Cardiac Catheters and Guidewires Market Size was valued at USD 15 Billion in 2023 and is expected to reach USD 29.36 billion by 2032, growing at a CAGR of 7.75% over the forecast period 2024-2032.
The Breastfeeding Accessories Market size was estimated at USD 2.55 billion in 2023 and is expected to reach USD 4.50 billion by 2032 with a growing CAGR of 6.5% during the forecast period of 2024-2032.
The Point-of-care Ultrasound Market size was USD 4.03 billion in 2023 and is expected to Reach USD 7.47 billion by 2032 and grow at a CAGR of 7.11% over the forecast period of 2024-2032.
The global Spatial Omics market, valued at USD 364.30 Million in 2023, is projected to reach USD 842.73 Million by 2032, growing at a compound annual growth rate CAGR of 10.30% during the forecast period.
The global tinnitus management market, valued at USD 3.45 Billion in 2023, is projected to reach USD 5.02 Billion by 2032, growing at a compound annual growth rate CAGR of 4.44% during the forecast period.
The Pharmacovigilance market valued USD 7.20 billion in 2023, and estimated to reach USD 18.52 billion by 2032 with CAGR 11.09% over the forecast period 2024-2032.
Hi! Click one of our member below to chat on Phone