The Intrapartum Monitoring Devices Market was valued at USD 2.34 billion in 2023 and is expected to reach USD 4.17 billion by 2032, growing at a CAGR of 6.69% from 2024-2032.
To Get more information on Intrapartum Monitoring Devices Market - Request Free Sample Report
The Intrapartum Monitoring Devices Market report provides original insights into fetal and maternal health incidence and prevalence in 2023, focusing on major risk factors and complications propelling device demand. The report also explores regional adoption and use patterns, with a complete analysis of geographical inequalities. It analyzes healthcare expenditure by region, decomposing the impact of government, commercial, private, and out-of-pocket spending on device adoption. Further, it also encompasses technological developments in monitoring devices and their uptake, as well as the increasing contribution of e-commerce and digital health platforms in increasing access to these devices.
The U.S. Intrapartum Monitoring Devices Market was valued at USD 0.73 billion in 2023 and is expected to reach USD 1.28 billion by 2032, growing at a CAGR of 6.41% from 2024-2032. The United States dominated the Intrapartum Monitoring Devices Market in North America on account of high rates of hospital births, widespread use of advanced monitoring technologies, and sophisticated healthcare infrastructure. Its supremacy is fueled further by stringent regulatory policies, rising maternal age, and the presence of top players like GE HealthCare and Philips Healthcare.
Drivers
The Rising Incidence of High-Risk Pregnancies is driving the market growth.
The incidence of high-risk pregnancies is on the rise globally as a result of rising maternal age, obesity, and chronic medical conditions. Conditions such as preeclampsia, gestational diabetes, and hypertension constitute high-risk pregnancies, which must be monitored carefully during labor to maintain the health of both mother and child. With an increase in such pregnancies, intrapartum monitoring devices are also in greater demand. These monitors are vital in offering real-time information, facilitating early detection of possible complications, and allowing timely medical intervention. Recent research indicates that the maternal mortality rate worldwide has improved as a result of advances in monitoring technology, resulting in improved outcomes for both mothers and infants. The increase in high-risk pregnancies especially fuels the market for non-invasive and continuous fetal monitoring solutions, also fueling the use of sophisticated intrapartum monitoring devices.
Technological advancements in monitoring devices are propelling the market growth.
Advances in technology in intrapartum monitoring equipment have transformed how healthcare professionals monitor the course of labor and evaluate fetal health. The incorporation of artificial intelligence (AI) and machine learning in these devices enables predictive analysis, which helps to detect possible complications like fetal distress or uterine contractions early in the labor process. In addition, the creation of wireless, portable, and wearable devices can provide better mobility for the mother during labor, which means more comfort without the loss of constant monitoring. These technologies offer real-time data that is precise and can be transmitted remotely, enhancing communication among medical teams. This connection also enables healthcare providers to make timely, well-informed decisions, potentially saving lives by preventing umbilical cord prolapse, fetal hypoxia, or obstructed labor. Further, contemporary intrapartum monitors are also progressively being provided with cloud-based facilities, which can store data and provide convenient access for long-term analysis, enabling both real-time decision-making as well as after-delivery management.
Restraint
The High Cost of Advanced Monitoring Systems is restraining the market growth.
One of the key restraining factors in the Intrapartum Monitoring Devices Market is the expensive nature of advanced monitoring devices. The devices are usually equipped with the latest technology, including wireless connectivity, artificial intelligence, and real-time analysis of data, which results in these devices being more expensive compared to conventional models. The price could be a huge hindrance for healthcare centers, particularly those operating in developing nations or small-sized healthcare facilities with limited financial resources. Although long-term gains from these sophisticated systems, including enhanced maternal and fetal health outcomes, could justify the initial expense, some institutions place other financial priorities ahead of acquiring expensive monitoring equipment. The cost factor may restrict the universal use of these devices, limiting their distribution to better-off hospitals or healthcare systems that have greater financial resources. Consequently, the market is hindered from achieving larger penetration, especially in low-income and mid-income nations.
Opportunities
Technological Advancements in Wireless and Remote Monitoring present a significant opportunity for market growth.
One of the key opportunities within the Intrapartum Monitoring Devices Market comes in the form of technological evolution in wireless and remote monitoring. As healthcare continues to move in a more patient-centered direction, demand for more mobile, non-invasive, and wireless-based monitoring has increased. Advances such as wearable intrapartum monitoring devices make it possible for ongoing monitoring of maternal and fetal well-being without confining the mother's movements. This creates the potential for the creation of more user-friendly, real-time monitoring systems that can enhance the labor experience. Beyond that, remote monitoring technologies provide expanded access to care, especially in underserved or rural communities where healthcare infrastructure is limited. Through these technologies, healthcare professionals can enhance patient outcomes, decrease the necessity for ongoing hospital visits, and ultimately reduce healthcare expenditures while improving patient satisfaction.
Challenges
One of the biggest issues confronting the Intrapartum Monitoring Devices Market is the complicated regulatory and compliance environment by geography.
In most countries, medical devices, especially those concerning maternal and fetal well-being, are subjected to rigorous approval and testing procedures before they are allowed on the market to sell. These processes differ according to region, making it a complicated situation for companies to deal with. Also, delays in approval from regulatory bodies and shifts in healthcare policy can cause production launch bottlenecks, constraining the growth of the market. The producers also need to see that their products are safe according to required standards and adhere to different standards of wireless connectivity and data privacy. These compliance barriers can cause delays in the process of innovation and raise the overall cost of product introduction into the market.
By Product
The Electrodes segment dominated the Intrapartum Monitoring Devices Market with a 56.30% market share in 2023 because of its critical application in non-invasive labor and delivery monitoring. Electrodes like fetal scalp electrodes and intrauterine pressure catheters are indispensable for precise real-time measurement of fetal heart rate (FHR) and uterine contractions, which are indispensable in determining labor progress and fetal health. These products are commonly used in maternity centers and hospitals because they can offer continuous, precise data, allowing timely interventions when needed. The extensive use of electrodes, combined with their relatively low cost and efficiency in offering necessary clinical data, was the reason behind the dominance of this segment. Moreover, their compatibility with multiple monitoring systems makes them even stronger in the market.
The Monitors segment will witness the fastest growth in the forecast years, with 6.82% CAGR, as the continued evolution of monitoring technology provides additional features such as wireless connectivity, real-time tracking of data, and connectivity with digital health platforms. These advancements assist in enhancing the monitoring process for healthcare providers as well as patients, thereby propelling adoption in all healthcare settings. In addition, with growing demand for more complex monitoring systems to evaluate both maternal and fetal health on an ongoing basis, the monitors segment is expected to grow at a high rate. Moreover, the increasing adoption of digital health technologies and e-health solutions has increased the demand for sophisticated monitoring devices that provide more accurate and real-time information about the labor process, thus driving market growth.
By End Use
The Hospitals segment dominated the intrapartum monitoring devices market with a 48.16% market share because of the large number of deliveries and critical care procedures offered in such facilities. Hospitals have sophisticated infrastructure and medical resources to handle complex and high-risk pregnancies, so they are the key end-users of intrapartum monitoring devices. The demand for precise, real-time tracking of fetal heart rate, uterine contractions, and maternal status during labor is most critical, and hospitals are generally well-equipped to spend on high-end machines to guarantee patient safety. Furthermore, hospitals are the most prevalent site for labor and delivery, adding to their leading market share. The widespread adoption of monitoring equipment in these facilities to minimize the risk of complications further entrenched the leadership of the hospitals segment.
The Maternity Centers segment will exhibit the fastest growth in the forecast period because of the increasing demand for specialized, individualized care during delivery in non-hospital settings. With an increased demand for less invasive and more comfortable birthing experiences, maternity centers are now more sought after as a destination for low-risk pregnancies and births. These centers are implementing high-tech intrapartum monitoring devices to improve the safety and health of both mothers and infants while preserving a more natural delivery setting. While maternity centers continue to expand and gain in popularity, especially within developed countries, demand for monitoring devices that can monitor maternal and fetal well-being during the labor process is expected to increase substantially. Outpatient-oriented care coupled with improvements in monitoring technologies will spur growth within the segment in the future.
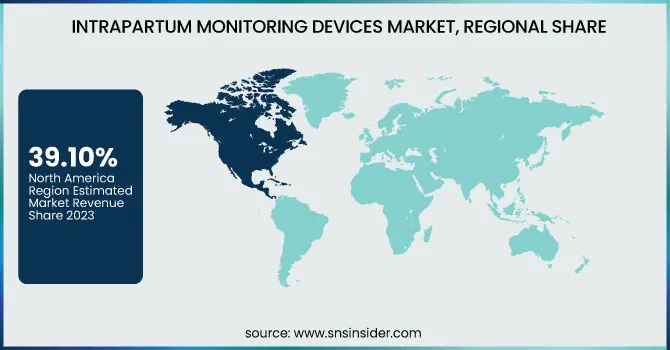
North America dominated the intrapartum monitoring devices market with a 39.10% market share in 2023, owing to its strong healthcare infrastructure, early embrace of advanced medical technology, and high level of awareness for maternal and fetal well-being. The region is aided by favorable government policies, high healthcare spending, and the strong presence of market leaders actively investing in research and development. Additionally, the increasing incidence of high-risk pregnancies and maternal age-related complications has necessitated a higher demand for intrapartum monitoring during labor and delivery. The United States, in particular, experiences extensive use of intrapartum monitoring devices in hospitals and maternity care centers due to stringent clinical protocols and insurance coverage, which makes it more accessible and affordable.
Asia Pacific is the fastest-growing region in the intrapartum monitoring devices market with 7.14% CAGR as a result of increasing birth rates, enhancing access to quality maternal care, and an increased emphasis on maternal and infant mortality reduction. Rapid urbanization, growing healthcare expenditures, and efforts by governments and international health agencies are promoting hospital and maternity clinic infrastructure development. These nations, like China, India, and Indonesia, are witnessing increasing demand for sophisticated monitoring devices in technology, fueled by an expanding middle class and increased awareness of prenatal and perinatal care. These are further spurred by private investment and foreign cooperation that are speeding up the transition toward improved technology in rural and semi-urban healthcare facilities.
Get Customized Report as per Your Business Requirement - Enquiry Now
Philips Healthcare (Avalon FM Series Fetal Monitors, IntelliVue MP Series Monitors)
GE HealthCare (Corometrics 250cx Fetal Monitor, Novii Wireless Patch System)
Medtronic plc (Nellcor Portable SpO₂ Patient Monitoring System, Capnostream 35 Portable Respiratory Monitor)
Mindray Medical International Limited (BeneVision N Series Monitors, iMEC12 Fetal Monitor)
Koninklijke N.V. (Royal Philips) (Avalon CL Base Station, IntelliSpace Perinatal)
Analogic Corporation (Sonicaid Team Fetal Monitor, Sonicaid FetalCare 3)
Natus Medical Incorporated (Embla Embletta MPR Sleep System, Inomed Neuro Monitoring)
Monica Healthcare Ltd. (Monica AN24, Novii Wireless Patch System)
Edan Instruments Inc. (F6 Express Fetal Monitor, iM70 Patient Monitor)
Bionet Co., Ltd. (FC1400 Fetal Monitor, BM3Vet Elite Monitor)
Spacelabs Healthcare (Evo Patient Monitor, Xprezzon Monitoring System)
Trismed Co., Ltd. (MT-610 Fetal Monitor, MP-1000 Multiparameter Monitor)
Schiller AG (Truscope Ultra Monitor, Argus LCM Plus)
Mediana Co., Ltd. (MT-830 Fetal Monitor, YM6000 Vital Signs Monitor)
Shenzhen Mindray Bio-Medical Electronics Co., Ltd. (MEC-1200, MFM-CMS Fetal Monitor)
Drägerwerk AG & Co. KGaA (Infinity Delta Monitor, Oxylog 3000 Plus)
Fukuda Denshi Co., Ltd. (FM-980 Fetal Monitor, DS-8500 Patient Monitor)
Welch Allyn (a Hillrom Company) (Connex Spot Monitor, Propaq CS Monitor)
Comen Medical Instruments Co., Ltd. (STAR8000E Patient Monitor, C30 Fetal Monitor)
Beijing Aeonmed Co., Ltd. (VG70 Ventilator Monitor, A8 Fetal Monitor)
Suppliers (These suppliers commonly provide sensors (e.g., ECG, temperature, SpO₂), microelectronics, and connectivity components essential for real-time monitoring, signal transmission, and accurate performance of intrapartum monitoring devices.) In the Intrapartum Monitoring Devices Market
TE Connectivity Ltd.
Texas Instruments Incorporated
Amphenol Corporation
Molex LLC (a Koch Industries company)
Honeywell International Inc.
Analog Devices, Inc.
Sensirion AG
Murata Manufacturing Co., Ltd.
STMicroelectronics N.V.
Omron Corporation
February 2024 – GE HealthCare announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Novii+ Wireless Patch Solution. Designed for both antepartum and intrapartum care, the device noninvasively monitors and displays fetal heart rate, maternal heart rate, and uterine activity, providing real-time data for clinical teams. Its wireless, belt-free design enhances maternal mobility, promoting a more comfortable labor experience.
August 2024 – Medtronic plc revealed it has received FDA approval for its Simplera continuous glucose monitor (CGM). As Medtronic’s first all-in-one, disposable CGM, Simplera is approximately half the size of its predecessors. The compact, discreet design streamlines insertion and eliminates the need for overtape, enhancing ease of use and wearability.
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | US$ 2.34 Billion |
| Market Size by 2032 | US$ 4.17 Billion |
| CAGR | CAGR of 6.69 % From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Product (Monitors, Electrodes) • By Electrode Type (Fetal Scalp Electrodes, Intrauterine Pressure Catheter, Transducer for FHR, Transducer for Uterine Contractions) • By End Use (Hospitals, Maternity Centers, Others) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | Philips Healthcare, GE HealthCare, Medtronic plc, Mindray Medical International Limited, Koninklijke N.V. (Royal Philips), Analogic Corporation, Natus Medical Incorporated, Monica Healthcare Ltd., Edan Instruments Inc., Bionet Co., Ltd., Spacelabs Healthcare, Trismed Co., Ltd., Schiller AG, Mediana Co., Ltd., Shenzhen Mindray Bio-Medical Electronics Co., Ltd., Drägerwerk AG & Co. KGaA, Fukuda Denshi Co., Ltd., Welch Allyn (a Hillrom Company), Comen Medical Instruments Co., Ltd., Beijing Aeonmed Co., Ltd., and other players. |
Ans: The Intrapartum Monitoring Devices Market is expected to grow at a CAGR of 6.69% from 2024-2032.
Ans: The Intrapartum Monitoring Devices Market was USD 2.34 billion in 2023 and is expected to reach USD 4.17 billion by 2032.
Ans: Technological advancements in monitoring devices are propelling the market growth.
Ans: The “Electrodes” segment dominated the Intrapartum Monitoring Devices Market
Ans: North America dominated the Intrapartum Monitoring Devices Market in 2023.
Table of Contents:
1. Introduction
1.1 Market Definition
1.2 Scope (Inclusion and Exclusions)
1.3 Research Assumptions
2. Executive Summary
2.1 Market Overview
2.2 Regional Synopsis
2.3 Competitive Summary
3. Research Methodology
3.1 Top-Down Approach
3.2 Bottom-up Approach
3.3. Data Validation
3.4 Primary Interviews
4. Market Dynamics Impact Analysis
4.1 Market Driving Factors Analysis
4.1.2 Drivers
4.1.2 Restraints
4.1.3 Opportunities
4.1.4 Challenges
4.2 PESTLE Analysis
5. Statistical Insights and Trends Reporting
5.1 Maternal and Fetal Health Incidence and Prevalence (2023)
5.2 Adoption and Usage Trends by Region (2023)
5.3 Healthcare Spending by Region (Government, Commercial, Private, Out-of-Pocket) (2023)
5.4 Technological Advancements and Adoption Rates (2023-2025)
5.5 E-commerce and Digital Health Trends (2023)
6. Competitive Landscape
6.1 List of Major Companies, By Region
6.2 Market Share Analysis, By Region
6.3 Product Benchmarking
6.3.1 Product specifications and features
6.3.2 Pricing
6.4 Strategic Initiatives
6.4.1 Marketing and promotional activities
6.4.2 Distribution and Supply Chain Strategies
6.4.3 Expansion plans and new product launches
6.4.4 Strategic partnerships and collaborations
6.5 Technological Advancements
6.6 Market Positioning and Branding
7. Intrapartum Monitoring Devices Market Segmentation, By Product
7.1 Chapter Overview
7.2 Monitors
7.2.1 Monitors Market Trends Analysis (2020-2032)
7.2.2 Monitors Market Size Estimates and Forecasts to 2032 (USD Billion)
7.2.3 Fetal Scalp Electrodes
7.2.3.1 Fetal Scalp Electrodes Market Trends Analysis (2020-2032)
7.2.3.2 Fetal Scalp Electrodes Market Size Estimates and Forecasts to 2032 (USD Billion)
7.2.4 Intrauterine Pressure Catheter
7.2.4.1 Intrauterine Pressure Catheter Market Trends Analysis (2020-2032)
7.2.4.2 Intrauterine Pressure Catheter Market Size Estimates and Forecasts to 2032 (USD Billion)
7.2.5 Transducer for FHR
7.2.5.1 Transducer for FHR Market Trends Analysis (2020-2032)
7.2.5.2 Transducer for FHR Market Size Estimates and Forecasts to 2032 (USD Billion)
7.2.6 Transducer for Uterine Contractions
7.2.6.1 Transducer for Uterine Contractions Market Trends Analysis (2020-2032)
7.2.6.2 Transducer for Uterine Contractions Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3 Electrodes
7.3.1 Electrodes Market Trends Analysis (2020-2032)
7.3.2 Electrodes Market Size Estimates and Forecasts to 2032 (USD Billion)
8. Intrapartum Monitoring Devices Market Segmentation, By End User
8.1 Chapter Overview
8.2 Hospitals
8.2.1 Hospitals Market Trends Analysis (2020-2032)
8.2.2 Hospitals Market Size Estimates and Forecasts to 2032 (USD Billion)
8.3 Maternity Centers
8.3.1 Maternity Centers Market Trends Analysis (2020-2032)
8.3.2 Maternity Centers Market Size Estimates and Forecasts to 2032 (USD Billion)
8.4 Others
8.4.1 Others Market Trends Analysis (2020-2032)
8.4.2 Others Market Size Estimates and Forecasts to 2032 (USD Billion)
9. Regional Analysis
9.1 Chapter Overview
9.2 North America
9.2.1 Trends Analysis
9.2.2 North America Intrapartum Monitoring Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.2.3 North America Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.2.4 North America Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.2.5 USA
9.2.5.1 USA Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.2.5.2 USA Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.2.6 Canada
9.2.6.1 Canada Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.2.6.2 Canada Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.2.7 Mexico
9.2.7.1 Mexico Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.2.7.2 Mexico Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.3 Europe
9.3.1 Eastern Europe
9.3.1.1 Trends Analysis
9.3.1.2 Eastern Europe Intrapartum Monitoring Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.3.1.3 Eastern Europe Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.3.1.4 Eastern Europe Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.3.1.5 Poland
9.3.1.5.1 Poland Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.3.1.5.2 Poland Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.3.1.6 Romania
9.3.1.6.1 Romania Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.3.1.6.2 Romania Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.3.1.7 Hungary
9.3.1.7.1 Hungary Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.3.1.7.2 Hungary Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.3.1.8 Turkey
9.3.1.8.1 Turkey Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.3.1.8.2 Turkey Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.3.1.9 Rest of Eastern Europe
9.3.1.9.1 Rest of Eastern Europe Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.3.1.9.2 Rest of Eastern Europe Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.3.2 Western Europe
9.3.2.1 Trends Analysis
9.3.2.2 Western Europe Intrapartum Monitoring Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.3.2.3 Western Europe Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.3.2.4 Western Europe Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.3.2.5 Germany
9.3.2.5.1 Germany Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.3.2.5.2 Germany Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.3.2.6 France
9.3.2.6.1 France Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.3.2.6.2 France Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.3.2.7 UK
9.3.2.7.1 UK Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.3.2.7.2 UK Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.3.2.8 Italy
9.3.2.8.1 Italy Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.3.2.8.2 Italy Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.3.2.9 Spain
9.3.2.9.1 Spain Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.3.2.9.2 Spain Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.3.2.10 Netherlands
9.3.2.10.1 Netherlands Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.3.2.10.2 Netherlands Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.3.2.11 Switzerland
9.3.2.11.1 Switzerland Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.3.2.11.2 Switzerland Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.3.2.12 Austria
9.3.2.12.1 Austria Healthcare Predictive Analytic Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.3.2.12.2 Austria Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.3.2.13 Rest of Western Europe
9.3.2.13.1 Rest of Western Europe Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.3.2.13.2 Rest of Western Europe Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.4 Asia Pacific
9.4.1 Trends Analysis
9.4.2 Asia Pacific Intrapartum Monitoring Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.4.3 Asia Pacific Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.4.4 Asia Pacific Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.4.5 China
9.4.5.1 China Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.4.5.2 China Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.4.6 India
9.4.5.1 India Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.4.5.2 India Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.4.5 Japan
9.4.5.1 Japan Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.4.5.2 Japan Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.4.6 South Korea
9.4.6.1 South Korea Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.4.6.2 South Korea Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.4.7 Vietnam
9.4.7.1 Vietnam Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.2.7.2 Vietnam Healthcare Predictive Analytic Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.4.8 Singapore
9.4.8.1 Singapore Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.4.8.2 Singapore Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.4.9 Australia
9.4.9.1 Australia Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.4.9.2 Australia Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.4.10 Rest of Asia Pacific
9.4.10.1 Rest of Asia Pacific Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.4.10.2 Rest of Asia Pacific Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.5 Middle East and Africa
9.5.1 Middle East
9.5.1.1 Trends Analysis
9.5.1.2 Middle East Intrapartum Monitoring Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.5.1.3 Middle East Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.5.1.4 Middle East Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.5.1.5 UAE
9.5.1.5.1 UAE Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.5.1.5.2 UAE Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.5.1.6 Egypt
9.5.1.6.1 Egypt Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.5.1.6.2 Egypt Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.5.1.7 Saudi Arabia
9.5.1.7.1 Saudi Arabia Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.5.1.7.2 Saudi Arabia Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.5.1.8 Qatar
9.5.1.8.1 Qatar Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.5.1.8.2 Qatar Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.5.1.9 Rest of Middle East
9.5.1.9.1 Rest of Middle East Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.5.1.9.2 Rest of Middle East Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.5.2 Africa
9.5.2.1 Trends Analysis
9.5.2.2 Africa Intrapartum Monitoring Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.5.2.3 Africa Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.5.2.4 Africa Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.5.2.5 South Africa
9.5.2.5.1 South Africa Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.5.2.5.2 South Africa Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.5.2.6 Nigeria
9.5.2.6.1 Nigeria Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.5.2.6.2 Nigeria Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.5.2.7 Rest of Africa
9.5.2.7.1 Rest of Africa Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.5.2.7.2 Rest of Africa Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.6 Latin America
9.6.1 Trends Analysis
9.6.2 Latin America Intrapartum Monitoring Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.6.3 Latin America Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.6.4 Latin America Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.6.5 Brazil
9.6.5.1 Brazil Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.6.5.2 Brazil Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.6.6 Argentina
9.6.6.1 Argentina Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.6.6.2 Argentina Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.6.7 Colombia
9.6.7.1 Colombia Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.6.7.2 Colombia Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
9.6.8 Rest of Latin America
9.6.8.1 Rest of Latin America Intrapartum Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.6.8.2 Rest of Latin America Intrapartum Monitoring Devices Market Estimates and Forecasts, by End-Use (2020-2032) (USD Billion)
10. Company Profiles
10.1 Philips Healthcare
10.1.1 Company Overview
10.1.2 Financial
10.1.3 Products/ Services Offered
110.1.4 SWOT Analysis
10.2 GE HealthCare
10.2.1 Company Overview
10.2.2 Financial
10.2.3 Products/ Services Offered
10.2.4 SWOT Analysis
10.3 Medtronic plc
10.3.1 Company Overview
10.3.2 Financial
10.3.3 Products/ Services Offered
10.3.4 SWOT Analysis
10.4 Mindray Medical International Limited
10.4.1 Company Overview
10.4.2 Financial
10.4.3 Products/ Services Offered
10.4.4 SWOT Analysis
10.5 Koninklijke N.V.
10.5.1 Company Overview
10.5.2 Financial
10.5.3 Products/ Services Offered
10.5.4 SWOT Analysis
10.6 Analogic Corporation
10.6.1 Company Overview
10.6.2 Financial
10.6.3 Products/ Services Offered
10.6.4 SWOT Analysis
10.7 Natus Medical Incorporated
10.7.1 Company Overview
10.7.2 Financial
10.7.3 Products/ Services Offered
10.7.4 SWOT Analysis
10.8 Monica Healthcare Ltd.
10.8.1 Company Overview
10.8.2 Financial
10.8.3 Products/ Services Offered
10.8.4 SWOT Analysis
10.9 Edan Instruments Inc.
10.9.1 Company Overview
10.9.2 Financial
10.9.3 Products/ Services Offered
10.9.4 SWOT Analysis
10.10 Bionet Co., Ltd.
10.9.1 Company Overview
10.9.2 Financial
10.9.3 Products/ Services Offered
10.9.4 SWOT Analysis
11. Use Cases and Best Practices
12. Conclusion
An accurate research report requires proper strategizing as well as implementation. There are multiple factors involved in the completion of good and accurate research report and selecting the best methodology to compete the research is the toughest part. Since the research reports we provide play a crucial role in any company’s decision-making process, therefore we at SNS Insider always believe that we should choose the best method which gives us results closer to reality. This allows us to reach at a stage wherein we can provide our clients best and accurate investment to output ratio.
Each report that we prepare takes a timeframe of 350-400 business hours for production. Starting from the selection of titles through a couple of in-depth brain storming session to the final QC process before uploading our titles on our website we dedicate around 350 working hours. The titles are selected based on their current market cap and the foreseen CAGR and growth.
The 5 steps process:
Step 1: Secondary Research:
Secondary Research or Desk Research is as the name suggests is a research process wherein, we collect data through the readily available information. In this process we use various paid and unpaid databases which our team has access to and gather data through the same. This includes examining of listed companies’ annual reports, Journals, SEC filling etc. Apart from this our team has access to various associations across the globe across different industries. Lastly, we have exchange relationships with various university as well as individual libraries.

Step 2: Primary Research
When we talk about primary research, it is a type of study in which the researchers collect relevant data samples directly, rather than relying on previously collected data. This type of research is focused on gaining content specific facts that can be sued to solve specific problems. Since the collected data is fresh and first hand therefore it makes the study more accurate and genuine.
We at SNS Insider have divided Primary Research into 2 parts.
Part 1 wherein we interview the KOLs of major players as well as the upcoming ones across various geographic regions. This allows us to have their view over the market scenario and acts as an important tool to come closer to the accurate market numbers. As many as 45 paid and unpaid primary interviews are taken from both the demand and supply side of the industry to make sure we land at an accurate judgement and analysis of the market.
This step involves the triangulation of data wherein our team analyses the interview transcripts, online survey responses and observation of on filed participants. The below mentioned chart should give a better understanding of the part 1 of the primary interview.

Part 2: In this part of primary research the data collected via secondary research and the part 1 of the primary research is validated with the interviews from individual consultants and subject matter experts.
Consultants are those set of people who have at least 12 years of experience and expertise within the industry whereas Subject Matter Experts are those with at least 15 years of experience behind their back within the same space. The data with the help of two main processes i.e., FGDs (Focused Group Discussions) and IDs (Individual Discussions). This gives us a 3rd party nonbiased primary view of the market scenario making it a more dependable one while collation of the data pointers.
Step 3: Data Bank Validation
Once all the information is collected via primary and secondary sources, we run that information for data validation. At our intelligence centre our research heads track a lot of information related to the market which includes the quarterly reports, the daily stock prices, and other relevant information. Our data bank server gets updated every fortnight and that is how the information which we collected using our primary and secondary information is revalidated in real time.

Step 4: QA/QC Process
After all the data collection and validation our team does a final level of quality check and quality assurance to get rid of any unwanted or undesired mistakes. This might include but not limited to getting rid of the any typos, duplication of numbers or missing of any important information. The people involved in this process include technical content writers, research heads and graphics people. Once this process is completed the title gets uploader on our platform for our clients to read it.
Step 5: Final QC/QA Process:
This is the last process and comes when the client has ordered the study. In this process a final QA/QC is done before the study is emailed to the client. Since we believe in giving our clients a good experience of our research studies, therefore, to make sure that we do not lack at our end in any way humanly possible we do a final round of quality check and then dispatch the study to the client.
Intrapartum Monitoring Devices Market Key Segments:
By Product
Monitors
Electrodes
Fetal Scalp Electrodes
Intrauterine Pressure Catheter
Transducer for FHR
Transducer for Uterine Contractions
By End Use
Hospitals
Maternity Centers
Others
Request for Segment Customization as per your Business Requirement: Segment Customization Request
Regional Coverage:
North America
US
Canada
Mexico
Europe
Eastern Europe
Poland
Romania
Hungary
Turkey
Rest of Eastern Europe
Western Europe
Germany
France
UK
Italy
Spain
Netherlands
Switzerland
Austria
Rest of Western Europe
Asia Pacific
China
India
Japan
South Korea
Vietnam
Singapore
Australia
Rest of Asia Pacific
Middle East & Africa
Middle East
UAE
Egypt
Saudi Arabia
Qatar
Rest of Middle East
Africa
Nigeria
South Africa
Rest of Africa
Latin America
Brazil
Argentina
Colombia
Rest of Latin America
Request for Country Level Research Report: Country Level Customization Request
Available Customization
With the given market data, SNS Insider offers customization as per the company’s specific needs. The following customization options are available for the report:
Detailed Volume Analysis
Criss-Cross segment analysis (e.g. Product X Application)
Competitive Product Benchmarking
Geographic Analysis
Additional countries in any of the regions
Customized Data Representation
Detailed analysis and profiling of additional market players
Paracetamol IV Market was valued at USD 792.89 million in 2023 and is expected to reach USD 1091.71 million by 2032, growing at a CAGR of 3.66% from 2024-2032.
Clinical Communication and Collaboration Market Size was valued at USD 2.49 billion in 2023 and is expected to reach USD 10.24 billion by 2032, growing at a CAGR of 17.02% over the forecast period 2024-2032.
Fill-Finish Manufacturing Market Size was valued at USD 15.4 Billion in 2023 and is expected to reach USD 33.5 Billion by 2032, growing at a CAGR of 9.1% over the forecast period 2024-2032.
The Epilepsy Device Market Size was valued at USD 0.75 billion in 2023 and is expected to reach USD 1.18 billion by 2032 and grow at a CAGR of 5.15% over the forecast period 2024-2032.
The Nasal Drug Delivery Market was valued at USD 75.03 billion in 2023 and is projected to reach USD 137.75 billion by 2032, growing at a steady CAGR of 7.00% from 2024 to 2032.
The Fluoroscopy Imaging Systems Market Size was valued at USD 1.99 billion in 2023 and is expected to reach USD 2.45 billion by 2032 and grow at a CAGR of 2.37% over the forecast period 2024-2032.
Hi! Click one of our member below to chat on Phone