To Get More Information on Immunohistochemistry Market - Request Sample Report
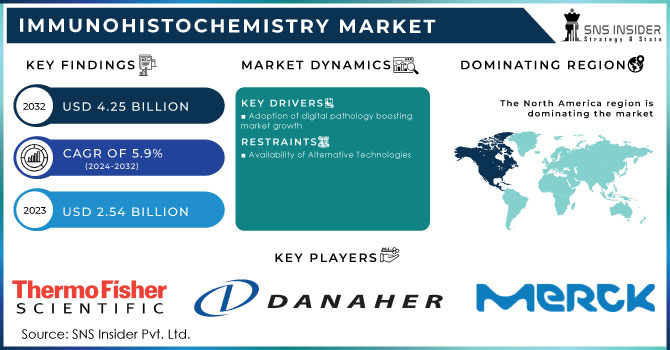
The Immunohistochemistry Market was valued at USD 2.49 billion in 2023 and is expected to reach USD 4.15 billion by 2032, growing at a CAGR of 5.85% over the forecast period of 2024-2032.
This report underscores increasing cancer incidence and prevalence by category, which continues to drive the need for IHC-based diagnostics. The report analyzes reagent and antibody use trends, including the growing usage of monoclonal and secondary antibodies as a result of their specificity and diagnostic precision. It also investigates pathology lab penetration and automation trends, where developed markets are quickly adopting digital pathology solutions. The healthcare and diagnostic expenditure trend by region is also examined in the report, taking into account government grants, private investments, and out-of-pocket spending in determining market access. Clinical trial activity with IHC biomarkers is increasing, especially in oncology studies, further underscoring the use of IHC within precision medicine strategies.
The U.S. Immunohistochemistry Market was valued at USD 0.78 billion in 2023 and is expected to reach USD 1.14 billion by 2032, growing at a CAGR of 4.35% over the forecast period of 2024-2032. In the United States, the IHC market is growing consistently with high cancer screening rates, good diagnostic infrastructure, and strong backing for clinical trials and precision medicine through federal support as well as from the private sector.
Market Dynamics
Drivers
The growing global cancer incidence and advancements in diagnostic precision are the primary forces driving demand for immunohistochemistry solutions.
The rise in cancer cases globally has become a major driving force for the immunohistochemistry market, considering the method's pivotal position in proper tumor classification and biomarker identification. There were an estimated 20 million new instances of cancer in 2023, as reported by the World Health Organization (WHO), a figure expected to increase dramatically over the next decade. IHC has a crucial role in diagnosing and subtyping cancer types like breast, prostate, and lung, where the treatment is directed by biomarkers such as HER2, ER/PR, and PD-L1. In addition, advances in IHC technology, e.g., automated staining instruments and multiplex IHC, are enhancing test reproducibility, throughput, and diagnostic accuracy. The combination of digital pathology and AI-based image analysis software is complementing efficiency in the clinical laboratory. With increasing personalized medicine and targeted treatments, the demand for companion diagnostics using IHC is quickly rising. Drug manufacturers are also applying IHC to drug development and clinical trials for patient stratification and evaluation of drug response. All these together fortify the market's growth momentum, making IHC a crucial support pillar in contemporary diagnostic and therapeutic practice.
Restraints
Despite its diagnostic value, immunohistochemistry faces adoption hurdles due to high costs, equipment needs, and skilled labor requirements.
The high operational expense of immunohistochemistry continues to be a major stumbling block, especially in low- and middle-income nations with scarce diagnostic infrastructure. Although advanced IHC systems bring the advantage of automation and precision, they do come at high capital expense and are beyond the reach of smaller laboratories and rural health facilities. Aside from expensive instruments, reagents and particularly good monoclonal antibodies are expensive and demand very stringent storage. Additionally, IHC methods are time-consuming and require skilled staff for optimal sample processing, staining, and interpretation. Irregularities in manual processes may also result in inter-observer variability, which compromises diagnostic quality. Small labs might not have the standardized procedures and skilled hands to provide reproducible results reliably, leading to the underutilization of IHC. Cost-to-benefit considerations become particularly relevant in non-urgent diagnostics, where quicker or less expensive alternatives might be preferable. Such cost and complexity considerations act in concert to limit the widespread use of IHC, particularly in facilities with poor financial or technical resources, even though its clinical value is established.
Opportunities
The rising demand for personalized therapies is creating strong growth potential for IHC as a tool for biomarker validation and treatment selection.
The trend toward personalized medicine opens up considerable growth prospects for the immunohistochemistry market. With targeted therapies becoming the norm, especially in oncology, there is a growing demand for companion diagnostics capable of identifying the right patients likely to benefit from certain treatments. IHC is at the center of this process by identifying protein biomarkers like HER2 in breast cancer or PD-L1 in non-small cell lung cancer that determine therapy decisions and outcomes. For instance, PD-L1 testing by IHC is a requirement for the administration of checkpoint inhibitors such as pembrolizumab. The FDA has cleared a few IHC-based companion diagnostics, with additional ones in the offing as pharma companies embark on biomarker-driven drug development. Moreover, advances in multiplex IHC and image analysis software are allowing scientists to assess several biomarkers simultaneously in a single tissue section, increasing efficiency and understanding. These technologies are especially promising for immuno-oncology and neurodegenerative disease studies. The increased interest in translational research, clinical trials, and precision diagnostics, complemented by robust R&D investment, provides a positive backdrop for IHC application expansion from cancer into infectious diseases, cardiovascular diseases, and beyond.
Challenges
Ensuring consistent, reproducible IHC results across different labs remains a persistent challenge affecting test reliability and clinical trust.
Despite technological progress, standardization in IHC is still a significant challenge, particularly when comparing results between laboratories and regions. Variability in staining protocols, quality of antibodies, tissue preparation, and interpretation can greatly affect diagnostic results. For example, variability in HER2 scoring can result in inappropriate treatment decisions in breast cancer patients. The absence of universal quality control standards and the use of manual techniques in most labs lead to variable results. Despite automation, variation in pre-analytical variables, e.g., fixation time, antigen retrieval, and reagent concentration, can bias results. These variations not only detract from diagnostic accuracy but also impede regulatory agencies' approval of new IHC assays and companion diagnostics. Moreover, inadequate access to comprehensive external quality assurance programs, particularly in developing countries, worsens reproducibility. AI image analysis and digital pathology both promise to limit human error but remain constrained by cost and integration considerations. Unless and until broad-scale harmonization of protocols and training occurs, the full clinical potential of IHC cannot be fulfilled, threatening significant market development and stakeholder confidence.
By Product
In 2023, the antibodies segment was the dominant contributor to the global immunohistochemistry market, with a substantial 43.4% share of the total revenue. This is primarily due to the critical role played by antibodies in detecting and visualizing certain antigens in tissue samples. Monoclonal and polyclonal antibodies are key elements in both diagnostic and research-oriented IHC applications, especially in oncology, infectious diseases, and autoimmune diseases. The growing use of companion diagnostics and personalized medicine, especially among cancer patients, has further driven the need for antibody-based testing, solidifying the segment's leadership position in the market.
On the other hand, the kits segment is expected to have the highest growth during the forecast period. This growth is expected to be driven by the increasing demand for ready-to-use, standardized testing solutions that simplify workflow efficiency and reduce human error. Kits provide a time-efficient and convenient method for laboratories by bundling all required reagents and antibodies in one package, improving reproducibility and consistency of test outcomes. The growing automation of IHC processes and increased demand for point-of-care diagnostic solutions are also likely to promote kit use, especially in resource-limited regions and developing economies looking to enhance diagnostic capabilities.
By Application
The diagnostics segment led the immunohistochemistry market in 2023, with a revenue share of more than 73% globally. The dominant position owes much to the irreplaceable role of IHC in clinical diagnostics, especially in oncology, where it is a gold standard for tumor identification and classification. IHC finds widespread application in detecting certain tumor markers, facilitating cancer type differentiation, grading, and prognosis. As the global cancer burden continues to increase, the demand for accurate and quick diagnostic measures has fueled the adoption of IHC on a large scale by hospitals and diagnostic facilities. The increasing requirement of companion diagnostics and biomarker testing in specific therapies also complements the dominance of the segment.
The research segment, on the other hand, is projected to grow at the most rapid rate throughout the forecast period. The growth in this segment is driven by increased investments in biotechnology, translational research, and life sciences, where IHC is central to the identification of disease mechanisms, drug target validation, and tissue-based investigations. Pharmaceutical industries and research organizations are increasingly relying on IHC to investigate drug efficacy and the pathogenesis of disease, particularly during preclinical stages and early clinical trials. The increasing incorporation of IHC in experimental pathology continues to make it more relevant in academic and industrial research.
By End-use
Hospitals and clinical diagnostic laboratories comprised the largest end-use segment within the global market for immunohistochemistry in 2023, accounting for over 74.7% of the revenue. This was mainly due to the large volume of IHC tests performed within clinical settings to diagnose diseases, monitor them, and stratify patients. These facilities are dependent on IHC to make correct pathological interpretations, especially for oncology patients, where biomarker identification and tumor categorization are very important for treatment planning. The availability of sophisticated infrastructure, expert pathologists, and organized workflows add to the dominance of hospitals and diagnostic laboratories in the IHC industry.
Conversely, research institutions are expected to expand at the highest rate over the forecasting period. This growth is due to the rising focus on biomedical research and studies incorporating tissue-based analysis. Since research institutions keep probing molecular and cellular disease mechanisms, IHC has emerged as a critical tool in confirming discoveries at the level of protein expression. The increased government and private financing, together with the partnerships between academia and pharmaceuticals, is also helping to grow IHC applications in research. Moreover, developments in multiplex IHC and digital pathology are improving research productivity and analytical potential in this space.
Regional Analysis
North America was the leading region in the immunohistochemistry market in 2023 due to its highly developed healthcare infrastructure, high use of personalized medicine, and high cancer incidence. Strong R&D spending, early adoption of technology-enabled IHC platforms, and a high number of FDA-approved companion diagnostics are strengths for the region. The presence of prominent market players and well-established clinical laboratories also reflects the maturity of the market in the U.S. and Canada. Government and private initiatives toward early cancer detection and precision oncology have also increased the demand for IHC-based diagnostics in hospitals and diagnostic centers.
The Asia Pacific region, however, is expected to be the fastest-growing market in the forecast period. This expansion is driven by a rapidly growing patient base, increasing cancer screening awareness, and growing healthcare spending in the likes of China, India, and South Korea. The WHO states that more than 50% of the world's cancer deaths now happen in Asia, which points toward an increasing need for enhanced diagnostic capacity. Government-initiated healthcare reforms, increasing investments in life sciences research, and the emergence of local diagnostic laboratories are opening up new avenues for IHC adoption in the region. Additionally, strategic partnerships between international companies and regional healthcare providers are driving market penetration and access to sophisticated IHC technologies.
Thermo Fisher Scientific Inc. – Lab Vision Antibodies, UltraVision Detection Systems, Histostain Kits
F. Hoffmann-La Roche Ltd. (Roche) – VENTANA BenchMark Series, VENTANA OptiView DAB IHC Detection Kit, VENTANA Primary Antibodies
Merck KGaA – Novocastra Antibodies, ImmunoDetector Detection System
Danaher Corporation – Bond IHC & ISH Stainers (via Leica Biosystems), Refine Detection Kits
PerkinElmer, Inc. – Opal Multiplex IHC Kits, TSA Detection Systems
Bio-Rad Laboratories, Inc. – StarBright Dyes, PrecisionAb Antibodies
Cell Signaling Technology, Inc. – IHC-validated Antibodies, SignalStain Detection Kits
Bio SB – PolyDetector HRP Systems, IHC Antibodies, SB-Multiplex Detection Kits
Agilent Technologies, Inc. – Dako Omnis, EnVision FLEX Detection Systems, Ready-to-Use Antibodies
Abcam plc – RabMAb Antibodies, SimpleStep ELISA Kits, IHC Primary Antibodies
Recent Developments
In Jan 2025, Roche received FDA approval for the label expansion of its PATHWAY anti-HER2/neu (4B5) Rabbit Monoclonal Primary Antibody as the first companion diagnostic to identify patients with HER2-ultralow metastatic breast cancer eligible for ENHERTU treatment. This approval enables the detection of a newly defined HER2-ultralow category, expanding targeted therapy options for HR-positive breast cancer patients.
| Report Attributes | Details |
| Market Size in 2023 | USD 2.49 billion |
| Market Size by 2032 | USD 4.15 billion |
| CAGR | CAGR of 5.85% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Product [Antibodies, Equipment, Reagents, Kits] • By Application [Diagnostics, Research] • By End-use [Hospitals & Diagnostic Laboratories, Research Institutes, Others] |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | Thermo Fisher Scientific Inc., F. Hoffmann-La Roche Ltd. (Roche), Merck KGaA, Danaher Corporation, PerkinElmer, Inc., Bio-Rad Laboratories, Inc., Cell Signaling Technology, Inc., Bio SB, Agilent Technologies, Inc., Abcam plc. |
Ans: Immunohistochemistry Market is anticipated to expand by 5.85% from 2024 to 2032.
Ans: USD 4.15 billion is expected to grow by 2032.
Ans: Immunohistochemistry Market size was valued at USD 2.49 billion in 2023.
Ans: Increasing demand for electronics devices and Increasing interest in power electronics
Ans: Rapid technological advancements in EMC Filtration.
Table of contents
1. Introduction
1.1 Market Definition
1.2 Scope (Inclusion and Exclusions)
1.3 Research & Academic Institutes Assumptions
2. Executive Summary
2.1 Market Overview
2.2 Regional Synopsis
2.3 Competitive Summary
3. Research & Academic Institutes Methodology
3.1 Top-Down Approach
3.2 Bottom-up Approach
3.3. Data Validation
3.4 Primary Interviews
4. Market Dynamics Impact Analysis
4.1 Market Driving Factors Analysis
4.1.1 Drivers
4.1.2 Restraints
4.1.3 Opportunities
4.1.4 Challenges
4.2 PESTLE Analysis
4.3 Porter’s Five Forces Model
5. Statistical Insights and Trends Reporting
5.1 Cancer Incidence and Prevalence, by Type (2023)
5.2 Reagent and Antibody Utilization Trends, by Type (Monoclonal, Polyclonal, Secondary Antibodies), 2023
5.3 Pathology Laboratory Penetration & Automation Trends, by Region (2023)
5.4 Healthcare & Diagnostic Spending Trends, by Region (Government, Private, Out-of-Pocket), 2023
5.5 Clinical Trial Activity Involving IHC Biomarkers (2020–2024)
6. Competitive Landscape
6.1 List of Major Companies, By Region
6.2 Market Share Analysis, By Region
6.3 Product Benchmarking
6.3.1 Product specifications and features
6.3.2 Pricing
6.4 Strategic Initiatives
6.4.1 Marketing and Promotional Activities
6.4.2 Distribution and Supply Chain Strategies
6.4.3 Expansion plans and new Product launches
6.4.4 Strategic partnerships and collaborations
6.5 Technological Advancements
6.6 Market Positioning and Branding
7. Immunohistochemistry Market Segmentation, by Product
7.1 Chapter Overview
7.2 Antibodies
7.2.1 Antibodies Market Trends Analysis (2020-2032)
7.2.2 Antibodies Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3 Equipment
7.3.1 Equipment Market Trends Analysis (2020-2032)
7.3.2 Equipment Market Size Estimates and Forecasts to 2032 (USD Billion)
7.4 Reagents
7.4.1 Reagents Market Trends Analysis (2020-2032)
7.4.2 Reagents Market Size Estimates and Forecasts to 2032 (USD Billion)
7.5 Kits
7.5.1 Kits Market Trends Analysis (2020-2032)
7.5.2 Kits Market Size Estimates and Forecasts to 2032 (USD Billion)
8. Immunohistochemistry Market Segmentation, by Application
8.1 Chapter Overview
8.2 Diagnostics
8.2.1 Diagnostics Market Trends Analysis (2020-2032)
8.2.2 Diagnostics Market Size Estimates and Forecasts to 2032 (USD Billion)
8.3 Research
8.3.1 Research Market Trends Analysis (2020-2032)
8.3.2 Research Market Size Estimates and Forecasts to 2032 (USD Billion)
9. Immunohistochemistry Market Segmentation, by End Use
9.1 Chapter Overview
9.2 Hospitals & Diagnostic Laboratories
9.2.1 Hospitals & Diagnostic Laboratories Market Trends Analysis (2020-2032)
9.2.2 Hospitals & Diagnostic Laboratories Market Size Estimates and Forecasts to 2032 (USD Billion)
9.3 Research Institutes
9.3.1 Research Institutes Market Trends Analysis (2020-2032)
9.3.2 Research Institutes Market Size Estimates and Forecasts to 2032 (USD Billion)
9.4 Others
9.4.1 Others Market Trends Analysis (2020-2032)
9.4.2 Others Market Size Estimates and Forecasts to 2032 (USD Billion)
10. Regional Analysis
10.1 Chapter Overview
10.2 North America
10.2.1 Trends Analysis
10.2.2 North America Immunohistochemistry Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.2.3 North America Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.2.4 North America Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.2.5 North America Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.2.6 USA
10.2.6.1 USA Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.2.6.2 USA Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.2.6.3 USA Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.2.7 Canada
10.2.7.1 Canada Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.2.7.2 Canada Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.2.7.3 Canada Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.2.8 Mexico
10.2.8.1 Mexico Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.2.8.2 Mexico Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.2.8.3 Mexico Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.3 Europe
10.3.1 Eastern Europe
10.3.1.1 Trends Analysis
10.3.1.2 Eastern Europe Immunohistochemistry Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.3.1.3 Eastern Europe Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.3.1.4 Eastern Europe Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.3.1.5 Eastern Europe Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.3.1.6 Poland
10.3.1.6.1 Poland Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.3.1.6.2 Poland Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.3.1.6.3 Poland Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.3.1.7 Romania
10.3.1.7.1 Romania Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.3.1.7.2 Romania Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.3.1.7.3 Romania Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.3.1.8 Hungary
10.3.1.8.1 Hungary Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.3.1.8.2 Hungary Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.3.1.8.3 Hungary Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.3.1.9 turkey
10.3.1.9.1 Turkey Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.3.1.9.2 Turkey Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.3.1.9.3 Turkey Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.3.1.10 Rest of Eastern Europe
10.3.1.10.1 Rest of Eastern Europe Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.3.1.10.2 Rest of Eastern Europe Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.3.1.10.3 Rest of Eastern Europe Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.3.2 Western Europe
10.3.2.1 Trends Analysis
10.3.2.2 Western Europe Immunohistochemistry Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.3.2.3 Western Europe Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.3.2.4 Western Europe Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.3.2.5 Western Europe Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.3.2.6 Germany
10.3.2.6.1 Germany Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.3.2.6.2 Germany Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.3.2.6.3 Germany Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.3.2.7 France
10.3.2.7.1 France Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.3.2.7.2 France Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.3.2.7.3 France Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.3.2.8 UK
10.3.2.8.1 UK Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.3.2.8.2 UK Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.3.2.8.3 UK Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.3.2.9 Italy
10.3.2.9.1 Italy Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.3.2.9.2 Italy Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.3.2.9.3 Italy Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.3.2.10 Spain
10.3.2.10.1 Spain Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.3.2.10.2 Spain Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.3.2.10.3 Spain Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.3.2.11 Netherlands
10.3.2.11.1 Netherlands Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.3.2.11.2 Netherlands Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.3.2.11.3 Netherlands Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.3.2.12 Switzerland
10.3.2.12.1 Switzerland Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.3.2.12.2 Switzerland Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.3.2.12.3 Switzerland Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.3.2.13 Austria
10.3.2.13.1 Austria Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.3.2.13.2 Austria Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.3.2.13.3 Austria Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.3.2.14 Rest of Western Europe
10.3.2.14.1 Rest of Western Europe Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.3.2.14.2 Rest of Western Europe Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.3.2.14.3 Rest of Western Europe Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.4 Asia Pacific
10.4.1 Trends Analysis
10.4.2 Asia Pacific Immunohistochemistry Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.4.3 Asia Pacific Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.4.4 Asia Pacific Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.4.5 Asia Pacific Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.4.6 China
10.4.6.1 China Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.4.6.2 China Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.4.6.3 China Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.4.7 India
10.4.7.1 India Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.4.7.2 India Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.4.7.3 India Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.4.8 Japan
10.4.8.1 Japan Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.4.8.2 Japan Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.4.8.3 Japan Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.4.9 South Korea
10.4.9.1 South Korea Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.4.9.2 South Korea Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.4.9.3 South Korea Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.4.10 Vietnam
10.4.10.1 Vietnam Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.4.10.2 Vietnam Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.4.10.3 Vietnam Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.4.11 Singapore
10.4.11.1 Singapore Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.4.11.2 Singapore Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.4.11.3 Singapore Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.4.12 Australia
10.4.12.1 Australia Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.4.12.2 Australia Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.4.12.3 Australia Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.4.13 Rest of Asia Pacific
10.4.13.1 Rest of Asia Pacific Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.4.13.2 Rest of Asia Pacific Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.4.13.3 Rest of Asia Pacific Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.5 Middle East and Africa
10.5.1 Middle East
10.5.1.1 Trends Analysis
10.5.1.2 Middle East Immunohistochemistry Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.5.1.3 Middle East Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.5.1.4 Middle East Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.5.1.5 Middle East Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.5.1.6 UAE
10.5.1.6.1 UAE Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.5.1.6.2 UAE Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.5.1.6.3 UAE Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.5.1.7 Egypt
10.5.1.7.1 Egypt Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.5.1.7.2 Egypt Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.5.1.7.3 Egypt Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.5.1.8 Saudi Arabia
10.5.1.8.1 Saudi Arabia Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.5.1.8.2 Saudi Arabia Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.5.1.8.3 Saudi Arabia Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.5.1.9 Qatar
10.5.1.9.1 Qatar Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.5.1.9.2 Qatar Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.5.1.9.3 Qatar Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.5.1.10 Rest of Middle East
10.5.1.10.1 Rest of Middle East Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.5.1.10.2 Rest of Middle East Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.5.1.10.3 Rest of Middle East Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.5.2 Africa
10.5.2.1 Trends Analysis
10.5.2.2 Africa Immunohistochemistry Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.5.2.3 Africa Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.5.2.4 Africa Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.5.2.5 Africa Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.5.2.6 South Africa
10.5.2.6.1 South Africa Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.5.2.6.2 South Africa Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.5.2.6.3 South Africa Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.5.2.7 Nigeria
10.5.2.7.1 Nigeria Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.5.2.7.2 Nigeria Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.5.2.7.3 Nigeria Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.5.2.8 Rest of Africa
10.5.2.8.1 Rest of Africa Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.5.2.8.2 Rest of Africa Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.5.2.8.3 Rest of Africa Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.6 Latin America
10.6.1 Trends Analysis
10.6.2 Latin America Immunohistochemistry Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.6.3 Latin America Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.6.4 Latin America Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.6.5 Latin America Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.6.6 Brazil
10.6.6.1 Brazil Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.6.6.2 Brazil Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.6.6.3 Brazil Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.6.7 Argentina
10.6.7.1 Argentina Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.6.7.2 Argentina Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.6.7.3 Argentina Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.6.8 Colombia
10.6.8.1 Colombia Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.6.8.2 Colombia Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.6.8.3 Colombia Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10.6.9 Rest of Latin America
10.6.9.1 Rest of Latin America Immunohistochemistry Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
10.6.9.2 Rest of Latin America Immunohistochemistry Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.6.9.3 Rest of Latin America Immunohistochemistry Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
11. Company Profiles
11.1 Thermo Fisher Scientific Inc.
11.1.1 Company Overview
11.1.2 Financial
11.1.3 Product / Services Offered
11.1.4 SWOT Analysis
11.2 F. Hoffmann-La Roche Ltd. (Roche)
11.2.1 Company Overview
11.2.2 Financial
11.2.3 Product / Services Offered
11.2.4 SWOT Analysis
11.3 Merck KGaA
11.3.1 Company Overview
11.3.2 Financial
11.3.3 Product / Services Offered
11.3.4 SWOT Analysis
11.4 Danaher Corporation
11.4.1 Company Overview
11.4.2 Financial
11.4.3 Product / Services Offered
11.4.4 SWOT Analysis
11.5 PerkinElmer, Inc.
11.5.1 Company Overview
11.5.2 Financial
11.5.3 Product / Services Offered
11.5.4 SWOT Analysis
11.6 Bio-Rad Laboratories, Inc.
11.6.1 Company Overview
11.6.2 Financial
11.6.3 Product / Services Offered
11.6.4 SWOT Analysis
11.7 Cell Signaling Technology, Inc.
11.7.1 Company Overview
11.7.2 Financial
11.7.3 Product / Services Offered
11.7.4 SWOT Analysis
11.8 Bio SB
11.8.1 Company Overview
11.8.2 Financial
11.8.3 Product / Services Offered
11.8.4 SWOT Analysis
11.9 Agilent Technologies, Inc.
11.9.1 Company Overview
11.9.2 Financial
11.9.3 Product / Services Offered
11.9.4 SWOT Analysis
11.10 Abcam plc
11.10.1 Company Overview
11.10.2 Financial
11.10.3 Product / Services Offered
11.10.4 SWOT Analysis
12. Use Cases and Best Practices
13. Conclusion
An accurate research report requires proper strategizing as well as implementation. There are multiple factors involved in the completion of good and accurate research report and selecting the best methodology to compete the research is the toughest part. Since the research reports we provide play a crucial role in any company’s decision-making process, therefore we at SNS Insider always believe that we should choose the best method which gives us results closer to reality. This allows us to reach at a stage wherein we can provide our clients best and accurate investment to output ratio.
Each report that we prepare takes a timeframe of 350-400 business hours for production. Starting from the selection of titles through a couple of in-depth brain storming session to the final QC process before uploading our titles on our website we dedicate around 350 working hours. The titles are selected based on their current market cap and the foreseen CAGR and growth.
The 5 steps process:
Step 1: Secondary Research:
Secondary Research or Desk Research is as the name suggests is a research process wherein, we collect data through the readily available information. In this process we use various paid and unpaid databases which our team has access to and gather data through the same. This includes examining of listed companies’ annual reports, Journals, SEC filling etc. Apart from this our team has access to various associations across the globe across different industries. Lastly, we have exchange relationships with various university as well as individual libraries.

Step 2: Primary Research
When we talk about primary research, it is a type of study in which the researchers collect relevant data samples directly, rather than relying on previously collected data. This type of research is focused on gaining content specific facts that can be sued to solve specific problems. Since the collected data is fresh and first hand therefore it makes the study more accurate and genuine.
We at SNS Insider have divided Primary Research into 2 parts.
Part 1 wherein we interview the KOLs of major players as well as the upcoming ones across various geographic regions. This allows us to have their view over the market scenario and acts as an important tool to come closer to the accurate market numbers. As many as 45 paid and unpaid primary interviews are taken from both the demand and supply side of the industry to make sure we land at an accurate judgement and analysis of the market.
This step involves the triangulation of data wherein our team analyses the interview transcripts, online survey responses and observation of on filed participants. The below mentioned chart should give a better understanding of the part 1 of the primary interview.

Part 2: In this part of primary research the data collected via secondary research and the part 1 of the primary research is validated with the interviews from individual consultants and subject matter experts.
Consultants are those set of people who have at least 12 years of experience and expertise within the industry whereas Subject Matter Experts are those with at least 15 years of experience behind their back within the same space. The data with the help of two main processes i.e., FGDs (Focused Group Discussions) and IDs (Individual Discussions). This gives us a 3rd party nonbiased primary view of the market scenario making it a more dependable one while collation of the data pointers.
Step 3: Data Bank Validation
Once all the information is collected via primary and secondary sources, we run that information for data validation. At our intelligence centre our research heads track a lot of information related to the market which includes the quarterly reports, the daily stock prices, and other relevant information. Our data bank server gets updated every fortnight and that is how the information which we collected using our primary and secondary information is revalidated in real time.

Step 4: QA/QC Process
After all the data collection and validation our team does a final level of quality check and quality assurance to get rid of any unwanted or undesired mistakes. This might include but not limited to getting rid of the any typos, duplication of numbers or missing of any important information. The people involved in this process include technical content writers, research heads and graphics people. Once this process is completed the title gets uploader on our platform for our clients to read it.
Step 5: Final QC/QA Process:
This is the last process and comes when the client has ordered the study. In this process a final QA/QC is done before the study is emailed to the client. Since we believe in giving our clients a good experience of our research studies, therefore, to make sure that we do not lack at our end in any way humanly possible we do a final round of quality check and then dispatch the study to the client.
Key Segments
By Product
Antibodies
Equipment
Reagents
Kits
By Application
Diagnostics
Research
By End-use
Hospitals & Diagnostic Laboratories
Research Institutes
Others
Request for Segment Customization as per your Business Requirement: Segment Customization Request
Regional Coverage:
North America
US
Canada
Mexico
Europe
Eastern Europe
Poland
Romania
Hungary
Turkey
Rest of Eastern Europe
Western Europe
Germany
France
UK
Italy
Spain
Netherlands
Switzerland
Austria
Rest of Western Europe
Asia Pacific
China
India
Japan
South Korea
Vietnam
Singapore
Australia
Rest of Asia Pacific
Middle East & Africa
Middle East
UAE
Egypt
Saudi Arabia
Qatar
Rest of Middle East
Africa
Nigeria
South Africa
Rest of Africa
Latin America
Brazil
Argentina
Colombia
Rest of Latin America
Request for Country Level Research Report: Country Level Customization Request
Available Customization
With the given market data, SNS Insider offers customization as per the company’s specific needs. The following customization options are available for the report:
Detailed Volume Analysis
Criss-Cross segment analysis (e.g. Product X Application)
Competitive Product Benchmarking
Geographic Analysis
Additional countries in any of the regions
Customized Data Representation
Detailed analysis and profiling of additional market players
The Host Cell Protein Testing Market Size was valued at USD 2.15 Bn in 2023 and will reach USD 4.34 Bn by 2032 and grow at a CAGR of 8.14% by 2024-2031.
The Liver Biopsy Market was valued at USD 0.90 billion in 2023 and is estimated to reach USD 1.65 billion by 2032, growing at a CAGR of 6.99% from 2024 to 2032.
The Transfer Membrane Market size was estimated at USD 402.91 million in 2023 and is expected to reach USD 645.1 million by 2032 with a growing CAGR of 4.9%.
The Medical Equipment Financing market size was USD 157.09 billion in 2023 and is expected to reach USD 305.98 billion by 2032 and grow at a CAGR of 7.69% over the forecast period of 2024-2032.
The Veterinary Surgical Instruments Market Size is projected to grow from USD 1.89 billion in 2023 to USD 3.10 billion by 2032, at a CAGR of 5.66%.
Epilepsy Drugs Market was valued at USD 7.6 billion in 2023 and is expected to reach USD 10.9 billion by 2032, growing at a CAGR of 4.06% from 2024-2032.
Hi! Click one of our member below to chat on Phone