Healthcare Contract Research Organization Market Size and Overview:
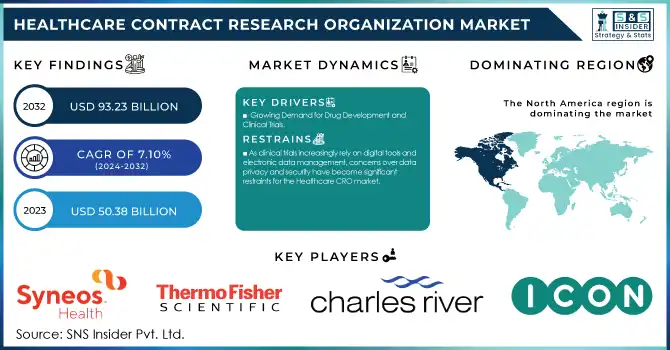
The Healthcare Contract Research Organization Market size was valued at USD 50.38 billion in 2023 and is projected to reach USD 93.23 billion by 2032, growing at a CAGR of 7.10% from 2024 to 2032.
Get more information on Healthcare CRO Market - Request Sample Report
The Healthcare Contract Research Organization (CRO) market is undergoing significant transformation, underpinned by rising investments in pharmaceutical R&D, increasing regulatory complexity, and the adoption of advanced technologies in clinical trials. As of 2024, the global CRO market is benefiting from a heightened demand for outsourced clinical services. For instance, IQVIA, one of the market leaders, reported an 11% year-over-year growth in its clinical services division, reflecting the industry’s robust demand for outsourced research services. This growth is driven by pharmaceutical companies’ need for greater efficiency, speed, and regulatory compliance.
The adoption of decentralized clinical trials (DCTs) has become a major trend, spurred by the COVID-19 pandemic and the need for more flexible, patient-centric approaches. By using digital tools, wearable devices, and telemedicine, DCTs enable the remote participation of patients, reducing the burden of travel and improving patient retention rates. According to Research, the use of digital technologies in clinical trials has increased by nearly 25% since 2020, improving operational efficiency and data quality. This shift is particularly important in chronic disease studies, where patient engagement and retention are key challenges.
Moreover, the growing focus on personalized medicine is also reshaping the CRO landscape. CROs are increasingly specializing in areas such as oncology, neurology, and ophthalmology, where clinical trials are often more complex and require tailored solutions. For example, Medpace has seen a surge in demand for its oncology trials, with a reported 35% increase in oncology-related revenue in 2023. This trend is fueled by an increase in the number of targeted therapies and biologics entering the market, which require specialized expertise in trial management, biomarker development, and patient recruitment.
Furthermore, regulatory challenges continue to drive the need for CROs. According to ClinicalTrials.gov, over 80% of clinical trials now face some form of regulatory delay, highlighting the importance of CROs in managing compliance. Companies like PPD are capitalizing on this by offering regulatory services across different regions, ensuring that trials meet the specific requirements of each market. These CROs leverage their deep understanding of global regulations to speed up the trial process and avoid costly setbacks.
Healthcare Contract Research Organization Market Dynamics
Drivers
-
Growing Demand for Drug Development and Clinical Trials
The escalating demand for new drugs and therapies, particularly in high-growth therapeutic areas such as oncology, rare diseases, and personalized medicine, is one of the primary drivers of the Healthcare CRO market. As pharmaceutical companies seek to innovate and introduce new treatments, the complexity of clinical trials increases, requiring specialized knowledge and infrastructure. Outsourcing clinical trials to CROs allows companies to leverage their expertise in managing clinical trials, ensuring regulatory compliance, and conducting research efficiently. This is particularly advantageous for small to medium-sized biotech firms, which may lack the necessary resources to conduct large-scale clinical studies in-house. CROs enable these companies to accelerate drug development timelines while managing costs, ultimately improving the time-to-market for new therapies and driving market growth.
-
The rapid adoption of technology in clinical trials has transformed the CRO market, enhancing operational efficiency, data accuracy, and patient recruitment.
Advanced technologies like artificial intelligence (AI), machine learning, and big data analytics are revolutionizing clinical research by enabling real-time monitoring, predictive analytics, and automated data management. AI and machine learning algorithms allow for faster patient recruitment, better screening processes, and more precise data analysis, all of which streamline the trial process. These technologies also help in managing large volumes of data generated from trials, improving overall data integrity. As CROs adopt these innovations, they can offer more efficient, cost-effective, and data-driven solutions to pharmaceutical companies, further boosting the demand for outsourcing services and contributing to the overall market growth.
-
Complex Regulatory Landscape and Compliance Needs
As the pharmaceutical industry becomes more globalized, the complexity of regulatory requirements continues to grow, necessitating expert guidance and compliance management. CROs play a crucial role in navigating these diverse and ever-evolving regulations across different regions, ensuring that clinical trials meet both local and international standards. With the increasing complexity of drug development, especially in emerging markets, pharmaceutical companies rely on CROs to manage regulatory hurdles, mitigate delays, and prevent costly setbacks. CROs with a deep understanding of the regulatory environment and experience in handling cross-border trials are in high demand, positioning themselves as key partners for drug developers looking to bring new treatments to market. This need for regulatory expertise is a significant driver of the CRO market's continued expansion.
Restraints
-
As clinical trials increasingly rely on digital tools and electronic data management, concerns over data privacy and security have become significant restraints for the Healthcare CRO market.
The sensitive nature of patient data collected during clinical trials makes it a prime target for cyberattacks, posing risks to patient confidentiality and regulatory compliance. Strict data protection regulations, such as the General Data Protection Regulation (GDPR) in Europe and similar laws globally, require CROs to implement robust cybersecurity measures to safeguard patient information. Failing to comply with these regulations can lead to hefty fines, reputational damage, and delays in trial progress. Additionally, the cost of maintaining secure data systems and adhering to these complex privacy requirements can be burdensome for CROs, especially smaller firms.
Healthcare Contract Research Organization Market Segment Insights
By Type
The Clinical Trials segment held the largest market share in 2023 with 73.2%, driven by the increasing demand for clinical development services in the pharmaceutical and biotech industries. As drug development advances, the need for comprehensive clinical trials spanning various phases—such as Phase I-IV—has surged. This segment is fueled by the expanding pipeline of drugs, particularly in oncology, rare diseases, and biologics. The complexity of clinical trials, regulatory requirements, and the need for patient recruitment strategies have made clinical trial services essential, further solidifying its dominance in the market.
The Pre-Clinical segment is expected to witness the fastest growth in the coming years. The pre-clinical phase, which involves laboratory testing and animal studies, is critical for assessing the safety and efficacy of potential new drugs. As pharmaceutical and biotech firms emphasize early-stage drug development, the demand for specialized preclinical services has increased. Additionally, the rise of advanced biotechnologies, such as gene therapy and cell therapy, has contributed to expanding the pre-clinical segment.
By Service
Clinical Monitoring services were the dominant segment in the Healthcare CRO market in 2023, accounting for 18.9% of the market share. This service involves overseeing the progress of clinical trials to ensure compliance with regulatory standards, assess patient safety, and monitor trial sites. The continued complexity of clinical trials and the increasing focus on patient-centric outcomes have elevated the importance of this service. Effective clinical monitoring is essential for the success of clinical trials, especially in complex therapeutic areas such as oncology, where close patient surveillance is critical.
Regulatory and Medical Affairs services have emerged as one of the fastest-growing segments in the CRO market, driven by the complexity and evolving nature of global regulatory frameworks. Pharmaceutical companies, particularly those operating across multiple regions, require specialized expertise to navigate the diverse regulatory landscapes, ensuring compliance and expediting drug approval processes. The increasing regulatory burden, including the need to address local and international standards, has led to heightened demand for these services, capturing a growing share of the market.
Healthcare Contract Research Organization Market Regional Outlook
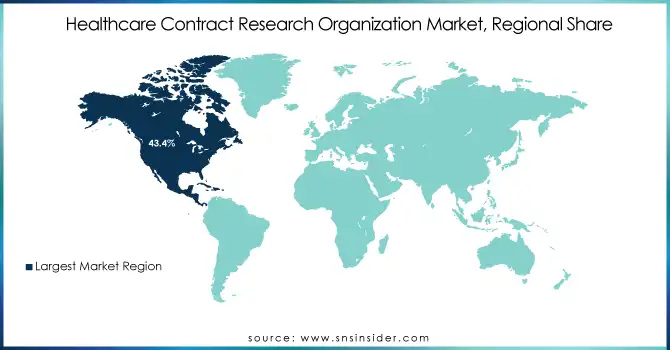
North America dominated the Healthcare Contract Research Organization (CRO) market in 2023 with a 43.4% share, driven by a robust pharmaceutical and biotechnology sector, high R&D investment, and a favorable regulatory environment. The United States leads the region, contributing significantly due to its advanced healthcare infrastructure, the presence of major CROs, and increasing clinical trial activities. North America also benefits from a strong focus on drug development for chronic diseases and oncology, which are high-priority therapeutic areas.
The Asia-Pacific region is the fastest-growing market, fueled by increasing clinical trial outsourcing to countries like China, India, and South Korea. Cost efficiency, a large patient pool for trials, and improvements in healthcare infrastructure make this region highly attractive for pharmaceutical companies. Additionally, government initiatives to promote clinical research and an expanding biotechnology sector further boost market growth.
Need any custom research on Healthcare CRO Market - Enquiry Now
Key Players:
-
ICON Plc (ICONIK AI Platform, Firecrest)
-
Charles River Laboratories (Early Discovery Services, InVivo Pharmacology Services)
-
Syneos Health (Synnovation Technology, Illingworth Research Group)
-
IQVIA Inc. (Orchestrated Clinical Trials (OCT), IQVIA Technologies)
-
GVK Biosciences Private Limited (Aragen) (Aragen Discovery Engine, CMC Services)
-
LabCorp (Covance) (Xcellerate Monitoring, Central Laboratory Services)
-
Parexel International Corporation (Parexel Biotech, Patient Innovation Center)
-
Thermo Fisher Scientific (BioServices, PPD Clinical Development)
-
CTI Clinical Trial & Consulting (Rare Disease Clinical Services, CTI Imaging Services)
-
PSI (PSI Clinical Trials, PSI Monitoring)
-
Medpace (Medpace Core Labs, ClinTrak Technology)
-
Ergomed (PrimeVigilance, Orphan Drug Development Services)
-
WuXi AppTec (WuXi Chemistry, WuXi Clinical Services)
-
Worldwide Clinical Trials (Bioanalytical Services, Early Phase Solutions)
-
Medidata Solutions, Inc. (Rave EDC, Clinical Cloud Solutions)
-
Pharmaron GMBH (Preclinical Research Solutions, Process R&D)
-
SGS SA (SGS Life Sciences, Biomarker Testing Solutions)
-
KCR S.A. (Trial Execution Services, Data Monitoring Solutions)
Recent Developments
In Jan 2025, Lindus Health successfully secured USD 55 million in Series B funding to enhance its clinical trial technology platform, aiming to streamline and modernize trial processes.
In Nov 2024, Research Grid secured USD 6.5 million to enhance its AI automation engine for clinical trials. The funding will support expansion into Asian and U.S. markets, advancement of AI algorithms, and workforce growth.
In Oct 2024, NAMSA and TERUMO entered a strategic outsourcing partnership to accelerate the global regulatory approval and commercialization of TERUMO's medical device portfolio through NAMSA's comprehensive clinical research, testing, and consulting services.
In July 2024, Emmes Group partnered with Miimansa AI to accelerate the adoption of generative AI in clinical research, acquiring Miimansa's advanced Clinical Entity Modeling tools based on large language modeling (LLM) techniques.
| Report Attributes | Details |
| Market Size in 2023 | USD 50.38 Billion |
| Market Size by 2032 | USD 93.23 Billion |
| CAGR | CAGR of 7.10% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Type [Drug Discovery (Target Validation, Lead Identification, Lead Optimization), Pre-Clinical, Clinical (Phase I Trial Services, Phase II Trial Services, Phase III Trial Services, Phase IV Trial Services)] • By Service [Project Management/Clinical Supply Management, Data Management, Regulatory/Medical Affairs, Medical Writing, Clinical Monitoring, Quality Management/ Assurance, Bio-statistics, Investigator Payments, Laboratory (Sterility Testing, Container/Closure Testing, Extractables and Leachable Testing, Environmental Monitoring (Including Microbiology Testing), Disinfectant Efficacy Studies, Others), Patient And Site Recruitment, Technology, Others] • By Therapeutic Area [Oncology, CNS Disorders, Infectious Diseases, Immunological Disorders, Cardiovascular Diseases, Respiratory Diseases, Diabetes, Ophthalmology, Pain Management, Others] • By Molecule [Pharmaceutical (Small Molecules, Biologics), Medical Device] |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | ICON Plc, Charles River Laboratories, Syneos Health, IQVIA Inc., GVK Biosciences Private Limited (Aragen), LabCorp, Parexel International Corporation, Thermo Fisher Scientific, CTI Clinical Trial & Consulting, PSI, Medpace, Ergomed, WuXi AppTec, Worldwide Clinical Trials, Medidata Solutions, Inc., Pharmaron GMBH, SGS SA, KCR S.A. |
| Key Drivers | • Growing Demand for Drug Development and Clinical Trials • The rapid adoption of technology in clinical trials has transformed the CRO market, enhancing operational efficiency, data accuracy, and patient recruitment. • Complex Regulatory Landscape and Compliance Needs |
| Restraints | • As clinical trials increasingly rely on digital tools and electronic data management, concerns over data privacy and security have become significant restraints for the Healthcare CRO market. |

