Get more information on Healthcare Cold Chain Monitoring Market - Request Sample Report
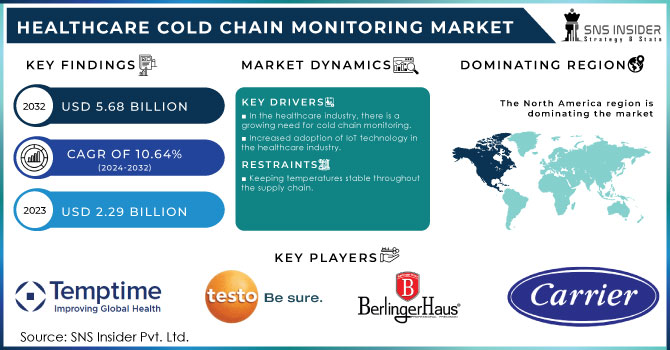
The Healthcare Cold Chain Monitoring Market Size was valued at USD 2.29 billion in 2023 and is expected to reach USD 5.68 billion by 2032 and grow at a CAGR of 10.64% over the forecast period 2024-2032.
Cold chain monitoring is the use of IoT technology to continuously monitor temperature-sensitive products being transported in a "cold chain" a supply chain of perishable and/or temperature-sensitive products such as pharmaceuticals, biologics, and food& beverages. If not properly monitored, suboptimal conditions during shipping and storage may degrade the quality of these products.
KEY DRIVERS:
In the healthcare industry, there is a growing need for cold chain monitoring.
Increased adoption of IoT technology in the healthcare industry.
RESTRAINTS:
Keeping temperatures stable throughout the supply chain.
OPPORTUNITIES:
Cold chain storage warehouse automation is in high demand.
Increased investment in the pharmaceutical cold chain.
CHALLENGES:
This will be covered under the final version of the report.
COVID-19 vaccines are transported safely with the help of IoT monitoring. According to the WHO, more than 40% of vaccines produced in developing countries each year are wasted due to flaws in the cold chain system. Cold chain shipping destroys 22% of temperature-sensitive biopharmaceutical products, including plasma. Excursions, or vaccines exposed to temperatures above the acceptable range. When it comes to storing or shipping vaccines or other biological items that can become inert if stored or shipped outside of a manufacturer's approved temperature or humidity range, hospitals, healthcare service providers, and logistics companies rely on refrigerated systems. This highlights the importance of cold chain monitoring systems in the healthcare industry. The ability to monitor these facilities and take corrective action in the event of a failure is critical to ensuring patient safety.
Based on the Component, the healthcare cold chain monitoring is segmented into Hardware and Software.
Based on Temperature, the healthcare cold chain monitoring is segmented into Chilled and Frozen. Certain drugs and biologics must be kept at specific temperatures, usually -25° to -10°C (-13° to 14°F), or "frozen," to remain effective. This includes, among other things, eye and ear drops, antibiotics, and injections. Cold chain providers ensure that shipments arrive at the proper temperature.
Based on Product, the healthcare cold chain monitoring is segmented into Biopharmaceuticals, Vaccines and Clinical Trial Materials. The vaccines segment held the greatest market share. Vaccines are biological products that can lose their potency if exposed to high temperatures or freezing temperatures.
Based on End User, the healthcare cold chain monitoring is segmented into Hospital & Clinics, Biopharmaceutical Companies and Research Institutes. Biopharmaceutical companies work with complex medicinal products made from living cells and/or organisms that have undergone advanced biotechnological processes.
BY PRODUCT
Clinical Trial Materials
BY COMPONENT
Hardware
Software
BY TEMPERATURE
Chilled
Frozen
BY END USER
Hospital & Clinics
Biopharmaceutical Companies
Research Institutes
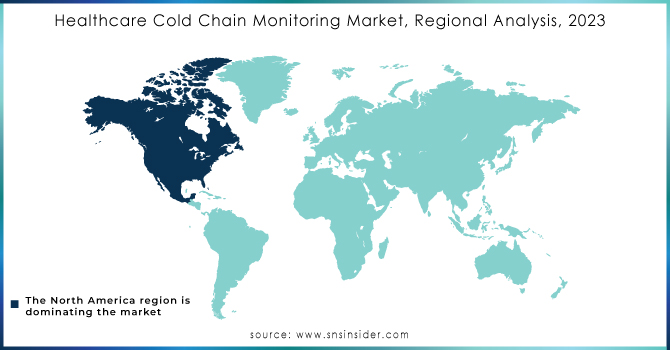
The market for healthcare cold chain monitoring has been researched in Asia-Pacific, North America, Europe, and the Rest of the World. North America held the largest market share. This can be attributed to the growing need for customised storage and transportation to ensure the effectiveness of healthcare products, as well as the region's growing emphasis on improving business agility.
The adoption of cold chain monitoring solutions by the midsize healthcare industry is expected to drive growth in the European healthcare cold chain monitoring market. The country's leading global cold chain monitoring innovator is constantly working to introduce new technologies.
Because of favourable government policies, the Chinese healthcare sector has expanded rapidly. China is an important player in the Asia-Pacific market due to its large population and increasing demand for cloud solutions by biopharmaceutical companies.
Need any customization research on Healthcare Cold Chain Monitoring Market - Enquiry Now
REGIONAL COVERAGE:
North America
USA
Canada
Mexico
Europe
Germany
UK
France
Italy
Spain
The Netherlands
Rest of Europe
Asia-Pacific
Japan
south Korea
China
India
Australia
Rest of Asia-Pacific
The Middle East & Africa
Israel
UAE
South Africa
Rest of Middle East & Africa
Latin America
Brazil
Argentina
Rest of Latin America
The key players in the healthcare cold chain monitoring market are Carrier, Berlinger, Testo SE, Signatrol, Temptime Corporation, Monnit Corporation, Emerson Electric, KGaA, Haier Biomedical and Cold Chain Technologies and other players.
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | US$ 2.29 Billion |
| Market Size by 2032 | US$ 5.68 Billion |
| CAGR |
CAGR of 10.64% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Product (Biopharmaceuticals, Vaccines, Clinical Trial Materials) • By Component (Hardware, Software) • By Temperature (Chilled, Frozen) • By End User (Hospital & Clinics, Biopharmaceutical Companies, Research Institutes) |
| Regional Analysis/Coverage | North America (USA, Canada, Mexico), Europe (Germany, UK, France, Italy, Spain, Netherlands, Rest of Europe), Asia-Pacific (Japan, South Korea, China, India, Australia, Rest of Asia-Pacific), The Middle East & Africa (Israel, UAE, South Africa, Rest of Middle East & Africa), Latin America (Brazil, Argentina, Rest of Latin America) |
| Company Profiles | Carrier, Berlinger, Testo SE, Signatrol, Temptime Corporation, Monnit Corporation, Emerson Electric, KGaA, Haier Biomedical and Cold Chain Technologies. |
| Key Drivers | • In the healthcare industry, there is a growing need for cold chain monitoring. • Increased adoption of IoT technology in the healthcare industry. |
| Restraints | • Keeping temperatures stable throughout the supply chain. |
Ans: The Healthcare Cold Chain Monitoring Market size was valued at 2.29 Bn in 2023.
The market value is expected to reach USD 5.68 billion by 2032.
The market has been segmented with respect to product, component, temperature and end-user.
Top-down research, bottom-up research, qualitative research, quantitative research, and Fundamental research.
Ans: The Healthcare Cold Chain Monitoring Market is growing at a CAGR of 10.64% over the forecast period 2024-2032.
Table of Contents
1. Introduction
1.1 Market Definition
1.2 Scope
1.3 Research Assumptions
2. Research Methodology
3. Market Dynamics
3.1 Drivers
3.2 Restraints
3.3 Opportunities
3.4 Challenges
4. Impact Analysis
4.1 COVID-19 Impact Analysis
4.2 Impact of Ukraine- Russia war
4.3 Impact of ongoing Recession
4.3.1 Introduction
4.3.2 Impact on major economies
4.3.2.1 US
4.3.2.2 Canada
4.3.2.3 Germany
4.3.2.4 France
4.3.2.5 United Kingdom
4.3.2.6 China
4.3.2.7 Japan
4.3.2.8 South Korea
4.3.2.9 Rest of the World
5. Value Chain Analysis
6. Porter’s 5 forces model
7. PEST Analysis
8. Healthcare Cold Chain Monitoring Market Segmentation, by product
8.1Introduction
8.2 Biopharmaceuticals
8.3 Vaccines
8.4 Clinical Trial Materials
9. Healthcare Cold Chain Monitoring Market Segmentation, by component
9.1Introduction
9.2 Hardware
9.3 Software
10. Healthcare Cold Chain Monitoring Market Segmentation, by temperature
10.1 Introduction
10.2 Chilled
10.3 Frozen
11. Healthcare Cold Chain Monitoring Market Segmentation, by end user
11.1 Introduction
11.2 Hospital & Clinics
11.3 Biopharmaceutical Companies
11.4 Research Institutes
12. Regional Analysis
12.1 Introduction
12.2 North America
12.2.1 USA
12.2.2 Canada
12.2.3 Mexico
12.3 Europe
12.3.1 Germany
12.3.2 UK
12.3.3 France
12.3.4 Italy
12.3.5 Spain
12.3.6 The Netherlands
12.3.7 Rest of Europe
12.4 Asia-Pacific
12.4.1 Japan
12.4.2 South Korea
12.4.3 China
12.4.4 India
12.4.5 Australia
12.4.6 Rest of Asia-Pacific
12.5 The Middle East & Africa
12.5.1 Israel
12.5.2 UAE
12.5.3 South Africa
12.5.4 Rest
12.6 Latin America
12.6.1 Brazil
12.6.2 Argentina
12.6.3 Rest of Latin America
13. Company Profiles
13.1 Berlinger
13.1.1 Financial
13.1.2 Products/ Services Offered
13.1.3 SWOT Analysis
13.1.4 The SNS view
13.2 Carrier
13.3 Testo SE
13.4 Signatrol
13.5 Temptime Corporation
13.6 Monnit Corporation
13.7 Emerson Electric
13.8 KGaA
13.9 Haier Biomedical
13.10 Cold Chain Technologies
14. Competitive Landscape
14.1 Competitive Benchmark
14.2 Market Share Analysis
14.3 Recent Developments
15. Conclusion
An accurate research report requires proper strategizing as well as implementation. There are multiple factors involved in the completion of good and accurate research report and selecting the best methodology to compete the research is the toughest part. Since the research reports we provide play a crucial role in any company’s decision-making process, therefore we at SNS Insider always believe that we should choose the best method which gives us results closer to reality. This allows us to reach at a stage wherein we can provide our clients best and accurate investment to output ratio.
Each report that we prepare takes a timeframe of 350-400 business hours for production. Starting from the selection of titles through a couple of in-depth brain storming session to the final QC process before uploading our titles on our website we dedicate around 350 working hours. The titles are selected based on their current market cap and the foreseen CAGR and growth.
The 5 steps process:
Step 1: Secondary Research:
Secondary Research or Desk Research is as the name suggests is a research process wherein, we collect data through the readily available information. In this process we use various paid and unpaid databases which our team has access to and gather data through the same. This includes examining of listed companies’ annual reports, Journals, SEC filling etc. Apart from this our team has access to various associations across the globe across different industries. Lastly, we have exchange relationships with various university as well as individual libraries.

Step 2: Primary Research
When we talk about primary research, it is a type of study in which the researchers collect relevant data samples directly, rather than relying on previously collected data. This type of research is focused on gaining content specific facts that can be sued to solve specific problems. Since the collected data is fresh and first hand therefore it makes the study more accurate and genuine.
We at SNS Insider have divided Primary Research into 2 parts.
Part 1 wherein we interview the KOLs of major players as well as the upcoming ones across various geographic regions. This allows us to have their view over the market scenario and acts as an important tool to come closer to the accurate market numbers. As many as 45 paid and unpaid primary interviews are taken from both the demand and supply side of the industry to make sure we land at an accurate judgement and analysis of the market.
This step involves the triangulation of data wherein our team analyses the interview transcripts, online survey responses and observation of on filed participants. The below mentioned chart should give a better understanding of the part 1 of the primary interview.

Part 2: In this part of primary research the data collected via secondary research and the part 1 of the primary research is validated with the interviews from individual consultants and subject matter experts.
Consultants are those set of people who have at least 12 years of experience and expertise within the industry whereas Subject Matter Experts are those with at least 15 years of experience behind their back within the same space. The data with the help of two main processes i.e., FGDs (Focused Group Discussions) and IDs (Individual Discussions). This gives us a 3rd party nonbiased primary view of the market scenario making it a more dependable one while collation of the data pointers.
Step 3: Data Bank Validation
Once all the information is collected via primary and secondary sources, we run that information for data validation. At our intelligence centre our research heads track a lot of information related to the market which includes the quarterly reports, the daily stock prices, and other relevant information. Our data bank server gets updated every fortnight and that is how the information which we collected using our primary and secondary information is revalidated in real time.

Step 4: QA/QC Process
After all the data collection and validation our team does a final level of quality check and quality assurance to get rid of any unwanted or undesired mistakes. This might include but not limited to getting rid of the any typos, duplication of numbers or missing of any important information. The people involved in this process include technical content writers, research heads and graphics people. Once this process is completed the title gets uploader on our platform for our clients to read it.
Step 5: Final QC/QA Process:
This is the last process and comes when the client has ordered the study. In this process a final QA/QC is done before the study is emailed to the client. Since we believe in giving our clients a good experience of our research studies, therefore, to make sure that we do not lack at our end in any way humanly possible we do a final round of quality check and then dispatch the study to the client.
The Lymphoma Treatment market size was USD 7.01 billion in 2023 and is expected to reach USD 14.66 billion by 2032 and grow at a CAGR of 8.54% over the forecast period of 2024-2032.
Metastatic Lung Adenocarcinoma Treatment Market was valued at USD 4.35 billion in 2023 and is expected to reach USD 11.58 billion by 2032, growing at a CAGR of 11.54% from 2024-2032.
The Immuno-oncology Clinical Trials Market was valued at USD 8.30 billion in 2023 and is expected to reach USD 23.63 billion by 2032, growing at a CAGR of 12.37% from 2024-2032.
The Contrast Media/Contrast Agent Market Size was valued at USD 6.50 Billion in 2023, and is expected to reach USD 12.28 Billion by 2032, and grow at a CAGR of 7.66%.
The Gene Therapy Market Size was valued at USD 9.2 Billion in 2023, and is expected to reach USD 54.39 Billion by 2032, and grow at a CAGR of 23.12%.
The Angiography Equipment Market Size was valued at USD 10.53 Billion in 2023 and is expected to reach USD 17.92 Billion by 2032 and grow at a CAGR of 6.11% over the forecast period 2024-2032.
Hi! Click one of our member below to chat on Phone