H1N1 (swine flu) Vaccination Market Size Analysis:
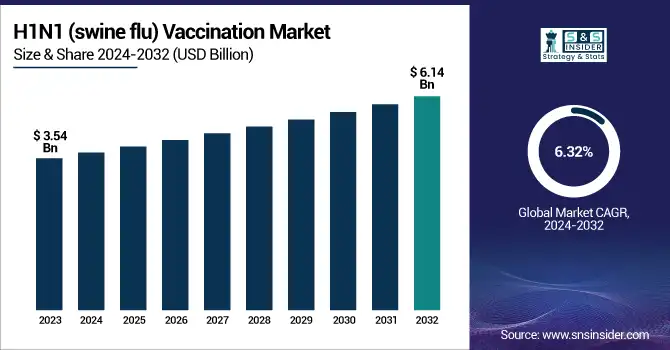
The H1N1 (swine flu) Vaccination Market was valued at USD 3.54 billion in 2023 and is expected to reach USD 6.14 billion by 2032, growing at a CAGR of 6.32% from 2024 to 2032.
Our H1N1 (Swine Flu) Vaccination Market report offers in-depth insights outside the scope of generic market growth. We have presented comprehensive 2023 incidence and prevalence statistics with a focus on the H1N1 burden by top regions. Our analysis includes regional vaccination coverage levels, giving readers an easy understanding of immunization programs. We investigate doses delivered and observe long-term distribution patterns. In addition, we evaluate government and private vaccination programs, exposing strategic investments in immunization efforts. Finally, our report provides supply and distribution patterns and reviews production capacity and global vaccine availability, providing a balanced market view.
To Get more information on H1N1 (swine flu) Vaccination Market - Request Free Sample Report
H1N1 (swine flu) Vaccination Market Dynamics
Drivers
-
Rising prevalence of influenza and pandemic threats accelerating the market growth.
The growing incidence of influenza outbreaks, such as H1N1 (swine flu), continues to fuel the need for vaccination. Seasonal influenza, as reported by the World Health Organization (WHO), causes about 3–5 million cases of severe illness worldwide each year, with pandemics such as H1N1 in 2009 further emphasizing the importance of effective vaccines. Governments and healthcare agencies are actively encouraging vaccination campaigns to avoid future outbreaks. Recent advances include the WHO's advocacy for new influenza vaccines that cover emerging viral strains, providing greater protection. Also, developments in vaccine manufacturing, including cell-based and recombinant technologies, have enhanced the speed and capacity of vaccine production. The constant emergence of new influenza strains also underscores the need for periodic immunization, hence the demand for H1N1 vaccines.
-
Advancements in vaccine development and distribution are propelling the market to grow.
Advances in vaccine technology have largely driven the development of the H1N1 vaccination market. The transition towards cell-based and mRNA vaccines, such as Moderna's mRNA-1010 influenza vaccine, is transforming immunization. The next-generation vaccines have better efficacy, faster production, and increased ability to accommodate changing influenza strains. Intranasal and needle-free vaccine products, like AstraZeneca's FluMist, are also offering ease of administration, thus improving patient compliance. In addition, large pharmaceutical firms are consolidating their supply chain and distribution networks, making vaccines accessible globally. For example, Seqirus increased production of its Flucelvax to address increasing demand in several regions. The enhanced R&D investment, coupled with global cooperation for pandemic preparedness, continues to drive market growth, providing wider and more effective vaccine coverage for H1N1 and other flu strains.
Restraint
-
Vaccine uncertainty and misinformation are restraining the market.
One of the key constraints in the H1N1 (swine flu) vaccine market is vaccine hesitancy, fueled by misinformation and lack of trust in vaccines. Despite robust scientific evidence for the safety and effectiveness of influenza vaccines, a large segment of the population is still hesitant to be vaccinated because of fears regarding side effects, effectiveness, and conspiracy theories. Vaccine hesitation is among the WHO's global health threats number one, they say. Dissemination of misinformation on social media has helped fuel skepticism and reduce vaccination uptake. Some believe that the influenza vaccine is not required because of fallacies regarding natural immunity. Vaccine hesitation hampers global efforts at immunization, making herd immunity difficult to attain. Combating this problem demands more robust public education campaigns, open communication from the health authorities, and policy measures to combat misinformation effectively.
Opportunities
-
Expansion of Universal Influenza Vaccination Programs creating opportunity for the H1N1 (swine flu) Vaccination Market.
Governments and health authorities across the globe are increasingly promoting universal influenza vaccination programs, which pose a great opportunity for the H1N1 vaccination market. Most nations have adopted policies to add influenza vaccines, such as H1N1, to routine immunization programs, especially among high-risk groups like pregnant women, children, and the elderly. The CDC, for example, still recommends annual influenza vaccinations for almost all persons above six months of age. Furthermore, pandemic preparedness funding has increased, with the likes of Gavi, WHO, and country health ministries funding mass immunization campaigns. Growth in national immunization programs, combined with improved vaccine storage and delivery systems, provides a strong platform for producers to raise production and deliver to more populations. This transformation to preventative care and government-sponsored vaccination campaigns should increase the market considerably.
Challenges
-
Supply Chain Constraints and Distribution Inefficiencies challenging the market.
One of the key issues in the H1N1 vaccine market is the complexity of supply chain management and vaccine distribution. All vaccines, including H1N1 vaccines, have a relatively short shelf life and need stringent cold chain logistics to be viable. In low- and middle-income countries, poor storage facilities, poor transportation infrastructure, and delays in the supply chain affect vaccine availability. The picture is compounded during pandemics with unforeseen spikes in demand that can cause bottlenecks in production and shortages. Moreover, global imbalances in access to vaccines still exist, with some parts of the world struggling to get enough doses. The COVID-19 pandemic brought to the fore weaknesses in international vaccine supply chains, placing a premium on enhanced logistics and production capacity. To overcome these issues, investments in infrastructure, improved forecasting tools, and global cooperation to provide vaccines on time are necessary.
H1N1 (swine flu) Vaccination Market Segmentation Analysis
By Type
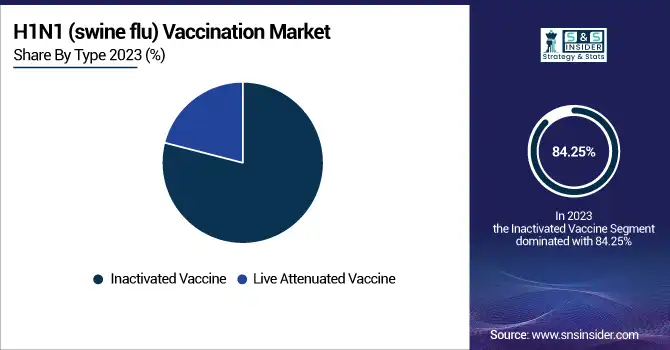
The inactivated vaccine segment dominated the H1N1 vaccination market with an 84.25 % market share in 2023, owing to its established safety profile, broad acceptance, and comprehensive utilization in national immunization programs. In contrast to live attenuated vaccines, inactivated vaccines consist of killed virus particles, which exclude the potential for causing infection, thus recommending themselves for immunocompromised persons, pregnant women, and geriatric populations. Regulatory bodies like the FDA, WHO, and CDC favor inactivated vaccines as the first line of immunization against the flu, adding to their demand. Moreover, major pharmaceutical corporations like Sanofi Pasteur, GlaxoSmithKline, and CSL Limited have made significant efforts to produce and distribute inactivated H1N1 vaccines. The ease of storage, transportation, and existing infrastructure for injectable vaccine delivery have also helped the segment dominate the market.
The live attenuated vaccine segment is expected to see the fastest growth during the forecast period because it can trigger a more robust and long-term immune response. In contrast to inactivated vaccines, live attenuated vaccines simulate natural infection by employing a weakened strain of the virus, which increases mucosal and cellular immunity. This vaccine is especially beneficial for pediatric immunization and mass vaccination campaigns because it is intranasally administered, which eliminates injections and enhances patient compliance. Advances in the formulation and manufacturing of vaccines in the recent past, along with mounting research on better stability and efficacy, are additionally propelling market adoption. Also, the increasing demand for needle-free vaccination technology and the increased emphasis on universal influenza vaccines will drive the live attenuated vaccine market to grow at a higher rate in the forecast years.
By Route of Administration
The injectable vaccine segment dominated the H1N1 (swine flu) vaccination market in 2023 because it is well-tested for efficacy, universally available, and highly accepted by healthcare practitioners. Intramuscular or subcutaneous intramuscular vaccines, which make up the majority of inactivated influenza vaccines, such as H1N1, are the regular selection in immunization programs for the country. Regulatory clearances from key health agencies like the FDA, WHO, and CDC have further supported the credibility of injectable vaccines as protectors against H1N1 infection. Moreover, the highly developed cold chain system and government-sponsored immunization programs have eased large-scale distribution. The position of injectable vaccines as first choices in high-risk groups like pregnant women, the elderly, and immunocompromised patients further consolidated their market leadership. The steady innovations of vaccine products by pharmaceutical firms, such as adjuvanted and high-dose injectable vaccines, also supported the leading position of the segment in 2023.
By Distribution Channel
The hospital pharmacy segment dominated the H1N1 (swine flu) vaccination market in 2023 with the extensive number of vaccine administrations in hospital settings, notably throughout outbreaks of flu and pandemic preparedness programs. Hospitals are the first points of contact for vaccinations, most notably for high-risk patients such as those with chronic disease, the immunocompromised, and pregnant women. Government-initiated vaccination programs and emergency stockpiling programs further escalated hospital pharmacies' involvement in distributing and administering H1N1 vaccines. Furthermore, hospitals possess improved storage facilities for temperature-sensitive vaccines to ensure proper handling and effectiveness. The availability of trained healthcare professionals within hospitals also enhances patient confidence in receiving vaccinations. Moreover, numerous nations employed hospital-based mass immunization programs to curb H1N1 outbreaks, further bolstering the segment's dominance in 2023.
H1N1 Vaccination Market Regional Insights
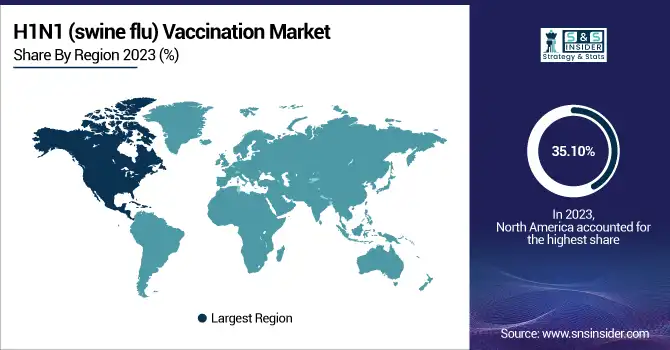
North America dominated the H1N1 vaccination market with around 35.10% market share in 2023 because of its established healthcare infrastructure, robust government immunization programs, and high levels of awareness about preventing influenza. The United States and Canada have widespread vaccination coverage, with organizations such as the CDC and Health Canada actively encouraging annual influenza immunization, including H1N1 strains. Also supporting regional supply and distribution is the presence of prominent vaccine producers Sanofi Pasteur, GlaxoSmithKline, and Seqirus. The region further has extensive government investment in pandemic preparedness through initiatives such as the Biomedical Advanced Research and Development Authority (BARDA), which supports the development and stockpiling of vaccines. High vaccination rates of seasonal influenza and aggressive public health policy mean North America remains dominant in the market.
Asia Pacific is experiencing the fastest growth in the H1N1 vaccination market with 7.41% CAGR throughout the forecast period, fueled by growing government campaigns, healthcare awareness, and an enormous target base. China, India, and Japan have widened their immunization programs considerably, incorporating influenza vaccines into national policy. The escalating incidence of influenza outbreaks in the region, in addition to urbanization and climate change, has increased the need for vaccines. Moreover, indigenous vaccine producers such as Serum Institute of India and Sinovac Biotech are ramping up production to make vaccines affordable and accessible. The region is also reaping benefits from higher investments in healthcare infrastructure and global partnerships for vaccine delivery. With a better regulatory environment and increasing vaccination coverage, Asia Pacific is likely to continue its robust growth trend in the years ahead.
Get Customized Report as per Your Business Requirement - Enquiry Now
Key Players in the H1N1 Vaccination Market
-
Sanofi Pasteur (Fluzone, Influenza A (H1N1) 2009 Monovalent Vaccine)
-
GlaxoSmithKline (GSK) (Pandemrix, Fluarix)
-
CSL Limited (Afluria, Influenza A (H1N1) 2009 Monovalent Vaccine)
-
Novartis Vaccines and Diagnostics (Focetria, Fluvirin)
-
MedImmune LLC (FluMist, Influenza A (H1N1) 2009 Monovalent Vaccine Live, Intranasal)
-
Baxter International Inc. (Celvapan, Preflucel)
-
Sinovac Biotech Ltd. (PANFLU.1, Anflu)
-
Hualan Biological Engineering Inc. (Hualan H1N1 Influenza Vaccine, Hualan Seasonal Influenza Vaccine)
-
Serum Institute of India Pvt. Ltd. (Nasovac, Influvac)
-
Zydus Cadila (VaxiFlu-S, VaxiFlu-4)
-
Bharat Biotech (HNVAC, BioVac-A)
-
Crucell N.V. (Inflexal V, FluCell)
-
Daiichi Sankyo Company, Limited (Kitasato Daiichi Sankyo Flu Vaccine, BE Flu)
-
Mitsubishi Tanabe Pharma Corporation (Influenza HA Vaccine, Influenza HA Vaccine "KMB")
-
Abbott Laboratories (Influvac, Prohibit)
-
Pfizer Inc. (Prevenar 13, Trumenba)
-
AstraZeneca (Fluenz, Vaxigrip)
-
Johnson & Johnson (Janssen H1N1 Vaccine, Ad26.ZEBOV)
-
Moderna, Inc. (mRNA-1010, mRNA-1273)
-
Seqirus (Flucelvax, Fluad)
Suppliers (These suppliers provide essential raw materials, bioprocessing solutions, contract manufacturing, and analytical testing services for the development and production of H1N1 and other influenza vaccines.) in H1N1 (swine flu) Vaccination Market.
-
Thermo Fisher Scientific
-
Merck KGaA
-
Lonza Group AG
-
Charles River Laboratories
-
Cytiva (Danaher Corporation)
-
Sartorius AG
-
FUJIFILM Diosynth Biotechnologies
-
Wuxi Biologics
-
AGC Biologics
-
Samsung Biologics
Recent Development in the H1N1 (swine flu) Vaccination Market
-
August 2023 – The Influenza A (H1N1) 2009 Monovalent vaccines have been approved by the U.S. FDA as a strain swap to current seasonal influenza vaccines. Due to extensive experience with manufacturing and developing seasonal influenza vaccines, these vaccines take a well-recognized history of safety and success in the United States. Those H1N1 vaccines approved will be subjected to the normal testing and lot release process, like seasonal influenza vaccines, to validate their quality and effectiveness before release.
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | US$ 3.54 billion |
| Market Size by 2032 | US$ 6.14 billion |
| CAGR | CAGR of 6.32% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Type (Inactivated Vaccine, Live Attenuated Vaccine) • By Route of Administration (Injectable Vaccine, Intranasal Vaccine) • By Distribution Channel (Retail Pharmacy, Hospital Pharmacy, Online Pharmacy) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | Sanofi Pasteur, GlaxoSmithKline (GSK), CSL Limited, Novartis Vaccines and Diagnostics, MedImmune LLC, Baxter International Inc., Sinovac Biotech Ltd., Hualan Biological Engineering Inc., Serum Institute of India Pvt. Ltd., Zydus Cadila, Bharat Biotech, Crucell N.V., Daiichi Sankyo Company, Limited, Mitsubishi Tanabe Pharma Corporation, Abbott Laboratories, Pfizer Inc., AstraZeneca, Johnson & Johnson, Moderna, Inc., Seqirus, and other players. |

