Get more information on Gene Therapy Market - Request Free Sample Report
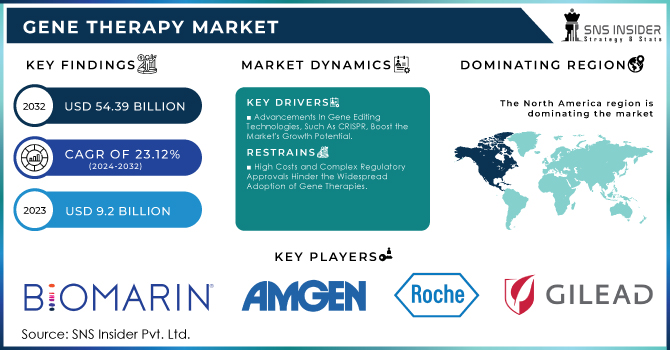
The Gene Therapy Market Size was valued at USD 9.2 Billion in 2023, and is expected to reach USD 54.39 Billion by 2032, and grow at a CAGR of 23.12%.
The gene therapy market has been rapidly growing in recent years due to significant advancements in biotechnology, genomics, and gene-editing technologies. Several gene therapies have received regulatory approval for specific genetic disorders, such as certain types of inherited blindness, spinal muscular atrophy, and rare immune deficiencies. The market potential for gene therapy is substantial, as it holds the promise of treating a wide range of genetic diseases that were previously considered incurable. However, it is essential to note that gene therapy is a complex and challenging field with various technical, regulatory, and ethical considerations. One of the major advantages of gene therapy products is that they are subject to rigorous regulatory approval before they can be marketed. They work at the genetic level, and their therapeutic effects are relatively more effective and longer-lasting than those of other traditional medicines. Due to this advantage, the number of approvals for gene therapy products is expected to increase. The US FDA has already approved over 10 products from 2021 to 2023, and there are a high number of products in the final stages of the clinical pipeline that will gain final approval and start marketing in the forecast period. Therefore, this is a major factor contributing to the growth of the market over the forecast period. Furthermore, the number of research and development activities for cell and gene therapy is increasing.
This is particularly the case because cell and gene therapy can mainly address problems associated with a specific group of incurable diseases. Therefore, it provides an opportunity for pharmaceutical and biotechnology companies to develop and market new types of cell and gene therapies. In addition to this, cell and gene therapies are the result of targeted therapy, which is one of the reasons they are widely adopted. Due to this reason, gene and cell therapies have been a source of treatment after reduction. However, repetition remains a threat, reducing the quality of patient treatment for long-term recovery.
Key Drivers:
The Increasing Prevalence of Genetic Disorders and Chronic Diseases Drives the Demand for Gene Therapy Solutions.
Advancements In Gene Editing Technologies, Such As CRISPR, Boost the Market's Growth Potential.
Restraints:
High Costs and Complex Regulatory Approvals Hinder the Widespread Adoption of Gene Therapies.
Limited Clinical Success Rates and Safety Concerns Pose Challenges to Market Expansion.
Opportunity:
Growing Investment In R&D For Novel Gene Therapies.
Expanding Applications in Oncology and Rare Diseases.
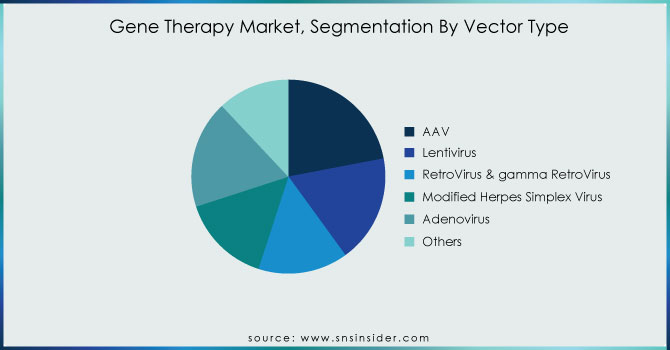
By Vector Type
The share of the AAV market segment was 22% in 2023. Several biopharmaceutical manufacturing firms have offered their viral vector platform for the manufacture of AAV gene therapy products. For example, a strategic research agreement to engineer novel adeno-associated virus AAV capsids that can deliver transformative gene therapy drugs that can address the root genetic issues that underlie various neuromuscular and central nervous system CNS disorders. The United States Department of Commerce’s National Institute of Standards and Technology NIST, the National Institute for Innovation in Manufacturing Biopharmaceuticals NIIMBL, and the United States Pharmacopeia announced a collaboration on AAV in July 2021. The trio will evaluate current analytical approaches and formulate procedures for AAV. As part of the agreement, multiple labs will perform an interlaboratory analysis of these vital quality characteristics, and the outcomes will be compared and analyzed. The exciting new gene therapy technologies will be boosted thanks to this collaborative effort, which will transform people’s lives.
Get Customized Report as per your Business Requirement - Request For Customized Report
By Indication
The spinal muscular atrophy segment dominated the market in 2023 by 21% of the market share. However, this disease is not frequent; it is one of the most common fatal inherited disorders of infancy. With the advent of Zolgensma AVXS-101, it has been evaluated that it is also effective in treating SMA, and constitutes a phenotypic transformation of the disorder. With this technology, the FDA gave its full approval to Novartis’ Zolgensma set to treat the primary cause of SMA. Zolgensma is also the only gene therapy product in this market. From recent findings, it can be inferred that the approval of Zolgensma types shows how the industry is increasingly utilizing therapies for severe inherited illnesses. Over the projection period, the Beta-Thalassemia Major/SCD segment is expected to register the fastest CAGR. SCD and β-thalassemia therapies are based on the transplantation of gene-modified hematopoietic stem cells. Clinical and preclinical trials show that this therapy is effective and safer.
However, its effectiveness is still compromised by other factors such as suboptimal gene expression levels & gene transfer efficiency, scarce stem-cell dosage and quality, and toxicity of myeloablative regimens. Also, the product was granted Orphan Drug status by the U.S. FDA for the management of patients with sickle cell disease. In addition to this, Vertex Pharmaceuticals also partnered with CRISPR Therapeutics in April 2021 for the development, production, and commercialization of CTX001 in sickle beta thalassemia and cell disease. The advancements in this segment are anticipated to play an essential role in driving the market’s growth in this segment.
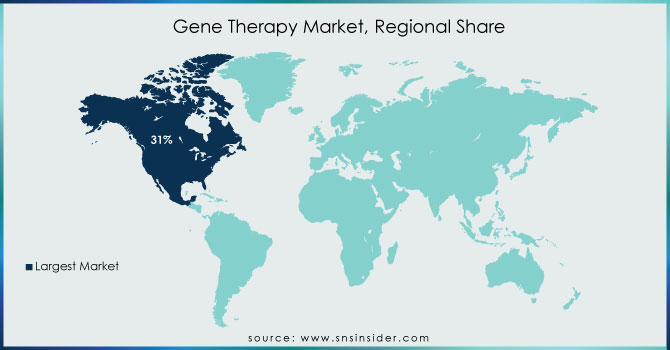
In terms of revenue, North America dominated the market with a 31% revenue share in 2023. During the forecast period, the region is expected to become the largest routine manufacturer of gene therapy in terms of approvals and revenue generated. An increasing number of investments by large and small companies for R&D to develop the ideal therapy drug and approval of gene therapy across the U.S. are expected to drive the market growth. For example, in 2020 and 2021, the NIH investment in Gene Therapy in the United States was USD 403 million and USD 481 million in 2020 and 2021 respectively. Over the estimated period, increasing investment in gene therapy in the United States will raise the development of gene therapies in this region, thus driving the growth of the market.
The key market players are BioMarin., Amgen Inc., F. Hoffmann-La Roche, Gilead Sciences, Inc., Merck & Co., Legend Biotech., Bristol-Myers Squibb Company, Sarepta Therapeutics, Inc., Novartis AG, uniQure N.V. and Other players.
In June 2023, U.S. FDA approved Sarepta for ELEVIDYS gene therapy to treat DMD in children of age 4-5 years
In January 2023, Voyager Therapeutics and Neurocrine Biosciences entered into a strategic collaboration for the commercialization & development of Voyager’s GBA1 program and other next-generation gene therapies for neurological diseases
In January 2023, Spark Therapeutics and Neurochase established a strategic collaboration to develop Neurochase’s unique delivery technology for use with selected gene treatments for rare disorders in the CNS. In this agreement, Neurochase will contribute its extensive knowledge in direct drug delivery technology to Spark’s premier AAV platform.
In January 2022, 64x Bio raises USD 55M in funding to advance their gene therapy manufacturing platform. With this initiative, the company is expected to expand its VectorSelect platform.
|
Report Attributes |
Details |
|---|---|
|
Market Size in 2023 |
US$ 9.20 Billion |
|
Market Size by 2032 |
US$ 54.39 Billion |
|
CAGR |
CAGR of 23.12% From 2024 to 2032 |
|
Base Year |
2023 |
|
Forecast Period |
2024-2032 |
|
Historical Data |
2020-2022 |
|
Report Scope & Coverage |
Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
|
Key Segments |
•By Indication (Acute Lymphoblastic Leukemia (ALL), Inherited Retinal Disease, Large B-cell lymphoma, Melanoma (lesions), Beta-Thalassemia Major/SCD, Spinal Muscular Atrophy (SMA) & Others) |
|
Regional Analysis/Coverage |
North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
|
Company Profiles |
BioMarin., Amgen Inc., F. Hoffmann-La Roche, Gilead Sciences, Inc., Merck & Co., Legend Biotech., Bristol-Myers Squibb Company, Sarepta Therapeutics, Inc., Novartis AG, uniQure N.V. and Other players |
|
Key Drivers |
•The Increasing Prevalence of Genetic Disorders and Chronic Diseases Drives the Demand for Gene Therapy Solutions. |
|
RESTRAINTS |
•High Costs and Complex Regulatory Approvals Hinder the Widespread Adoption of Gene Therapies. |
Ans. The Compound Annual Growth rate for Gene Therapy Market over the forecast period is 23.12%.
Ans. USD 54.39 Billion is the projected Gene Therapy market size of the market by 2032.
Ans. The forthcoming trends in the Gene Therapy Market include increased funding for R&D activities related to gene therapy and increased awareness of gene therapy, which will drive worldwide market growth. Furthermore, increased government funding, ethical acceptance of gene therapy for cancer treatment, and an increase in cancer prevalence promote market expansion.
Ans. The largest geographical market for Gene Therapy is North America.
Ans. The Gene Therapy Market includes the following countries: the United States, Canada, Mexico, Germany, France, the United Kingdom, Italy, Russia, Spain, the Netherlands, Switzerland, Belgium, Turkey, the Rest of Europe, China, Japan, India, Australia, South Korea, Singapore, Malaysia, and others.
Table of Contents
1. Introduction
1.1 Market Definition
1.2 Scope (Inclusion and Exclusions)
1.3 Research Assumptions
2. Executive Summary
2.1 Market Overview
2.2 Regional Synopsis
2.3 Competitive Summary
3. Research Methodology
3.1 Top-Down Approach
3.2 Bottom-up Approach
3.3. Data Validation
3.4 Primary Interviews
4. Market Dynamics Impact Analysis
4.1 Market Driving Factors Analysis
4.1.1 Drivers
4.1.2 Restraints
4.1.3 Opportunities
4.1.4 Challenges
4.2 PESTLE Analysis
4.3 Porter’s Five Forces Model
5. Statistical Insights and Trends Reporting
5.1 Incidence and Prevalence (2023)
5.2 Prescription Trends, (2023), by Region
5.3 Drug Volume: Indication and usage volumes of pharmaceuticals.
5.4 Healthcare Spending: Expenditure data by government, insurers, and out-of-pocket by patients
6. Competitive Landscape
6.1 List of Major Companies, By Region
6.2 Market Share Analysis, By Region
6.3 Indication Benchmarking
6.3.1 Indication specifications and features
6.3.2 Pricing
6.4 Strategic Initiatives
6.4.1 Marketing and promotional activities
6.4.2 Distribution and Supply Chain Strategies
6.4.3 Expansion plans and new Indication launches
6.4.4 Strategic partnerships and collaborations
6.5 Technological Advancements
6.6 Market Positioning and Branding
7. Gene Therapy Market Segmentation, by Indication
7.1 Chapter Overview
7.2 Acute Lymphoblastic Leukemia (ALL)
7.2.1 Acute Lymphoblastic Leukemia (ALL) Market Trends Analysis (2020-2032)
7.2.2 Acute Lymphoblastic Leukemia (ALL) Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3 Inherited Retinal Disease
7.3.1 Inherited Retinal Disease Market Trends Analysis (2020-2032)
7.3.2 Inherited Retinal Disease Market Size Estimates and Forecasts to 2032 (USD Billion)
7.4 Large B-Cell Lymphoma
7.4.1 Large B-Cell Lymphoma Market Trends Analysis (2020-2032)
7.4.2 Large B-Cell Lymphoma Market Size Estimates and Forecasts to 2032 (USD Billion)
7.5 Melanoma (lesions)
7.5.1 Melanoma (lesions) Market Trends Analysis (2020-2032)
7.5.2 Melanoma (lesions) Market Size Estimates and Forecasts to 2032 (USD Billion)
7.6 Beta-Thalassemia Major/SCD
7.6.1 Beta-Thalassemia Major/SCD Market Trends Analysis (2020-2032)
7.6.2 Beta-Thalassemia Major/SCD Market Size Estimates and Forecasts to 2032 (USD Billion)
7.7 Spinal Muscular Atrophy (SMA)
7.7.1 Spinal Muscular Atrophy (SMA) Market Trends Analysis (2020-2032)
7.7.2 Spinal Muscular Atrophy (SMA) Market Size Estimates and Forecasts to 2032 (USD Billion)
7.8 Others
7.8.1 Others Market Trends Analysis (2020-2032)
7.8.2 Others Market Size Estimates and Forecasts to 2032 (USD Billion)
8. Gene Therapy Market Segmentation, by Route of Administration
8.1 Chapter Overview
8.2 Intravenous
8.2.1 Intravenous Market Trends Analysis (2020-2032)
8.2.2 Intravenous Size Estimates and Forecasts to 2032 (USD Billion)
8.3 Others
8.3.1 Others Market Trends Analysis (2020-2032)
8.3.2 Others Market Size Estimates and Forecasts to 2032 (USD Billion)
9. Gene Therapy Market Segmentation, by Vector Type
9.1 Chapter Overview
9.2 Lentivirus
9.2.1 Lentivirus Market Trends Analysis (2020-2032)
9.2.2 Lentivirus Market Size Estimates and Forecasts to 2032 (USD Billion)
9.3 AAV
9.3.1 AAV Market Trends Analysis (2020-2032)
9.3.2 AAV Market Size Estimates and Forecasts to 2032 (USD Billion)
9.4 RetroVirus & gamma RetroVirus
9.4.1. RetroVirus & gamma RetroVirus Market Trends Analysis (2020-2032)
9.4.2 RetroVirus & gamma RetroVirus Market Size Estimates and Forecasts to 2032 (USD Billion)
9.5 Modified Herpes Simplex Virus
9.5.1 Modified Herpes Simplex Virus Market Trends Analysis (2020-2032)
9.5.2 Modified Herpes Simplex Virus Market Size Estimates and Forecasts to 2032 (USD Billion)
9.6 Adenovirus
9.6.1 Adenovirus Market Trends Analysis (2020-2032)
9.6.2 Adenovirus Market Size Estimates and Forecasts to 2032 (USD Billion)
9.7 Others
9.7.1 Others Market Trends Analysis (2020-2032)
9.7.2 Others Market Size Estimates and Forecasts to 2032 (USD Billion)
10. Regional Analysis
10.1 Chapter Overview
10.2 North America
10.2.1 Trends Analysis
10.2.2 North America Gene Therapy Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.2.3 North America Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.2.4 North America Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.2.5 North America Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.2.6 USA
10.2.6.1 USA Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.2.6.2 USA Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.2.6.3 USA Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.2.7 Canada
10.2.7.1 Canada Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.2.7.2 Canada Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.2.7.3 Canada Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.2.8 Mexico
10.2.8.1 Mexico Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.2.8.2 Mexico Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.2.8.3 Mexico Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.3 Europe
10.3.1 Eastern Europe
10.3.1.1 Trends Analysis
10.3.1.2 Eastern Europe Gene Therapy Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.3.1.3 Eastern Europe Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.3.1.4 Eastern Europe Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.3.1.5 Eastern Europe Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.3.1.6 Poland
10.3.1.6.1 Poland Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.3.1.6.2 Poland Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.3.1.6.3 Poland Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.3.1.7 Romania
10.3.1.7.1 Romania Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.3.1.7.2 Romania Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.3.1.7.3 Romania Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.3.1.8 Hungary
10.3.1.8.1 Hungary Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.3.1.8.2 Hungary Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.3.1.8.3 Hungary Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.3.1.9 Turkey
10.3.1.9.1 Turkey Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.3.1.9.2 Turkey Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.3.1.9.3 Turkey Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.3.1.10 Rest of Eastern Europe
10.3.1.10.1 Rest of Eastern Europe Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.3.1.10.2 Rest of Eastern Europe Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.3.1.10.3 Rest of Eastern Europe Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.3.2 Western Europe
10.3.2.1 Trends Analysis
10.3.2.2 Western Europe Gene Therapy Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.3.2.3 Western Europe Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.3.2.4 Western Europe Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.3.2.5 Western Europe Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.3.2.6 Germany
10.3.2.6.1 Germany Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.3.2.6.2 Germany Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.3.2.6.3 Germany Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.3.2.7 France
10.3.2.7.1 France Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.3.2.7.2 France Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.3.2.7.3 France Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.3.2.8 UK
10.3.2.8.1 UK Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.3.2.8.2 UK Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.3.2.8.3 UK Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.3.2.9 Italy
10.3.2.9.1 Italy Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.3.2.9.2 Italy Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.3.2.9.3 Italy Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.3.2.10 Spain
10.3.2.10.1 Spain Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.3.2.10.2 Spain Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.3.2.10.3 Spain Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.3.2.11 Netherlands
10.3.2.11.1 Netherlands Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.3.2.11.2 Netherlands Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.3.2.11.3 Netherlands Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.3.2.12 Switzerland
10.3.2.12.1 Switzerland Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.3.2.12.2 Switzerland Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.3.2.12.3 Switzerland Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.3.2.13 Austria
10.3.2.13.1 Austria Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.3.2.13.2 Austria Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.3.2.13.3 Austria Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.3.2.14 Rest of Western Europe
10.3.2.14.1 Rest of Western Europe Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.3.2.14.2 Rest of Western Europe Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.3.2.14.3 Rest of Western Europe Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.4 Asia Pacific
10.4.1 Trends Analysis
10.4.2 Asia Pacific Gene Therapy Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.4.3 Asia Pacific Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.4.4 Asia Pacific Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.4.5 Asia Pacific Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.4.6 China
10.4.6.1 China Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.4.6.2 China Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.4.6.3 China Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.4.7 India
10.4.7.1 India Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.4.7.2 India Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.4.7.3 India Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.4.8 Japan
10.4.8.1 Japan Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.4.8.2 Japan Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.4.8.3 Japan Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.4.9 South Korea
10.4.9.1 South Korea Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.4.9.2 South Korea Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.4.9.3 South Korea Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.4.10 Vietnam
10.4.10.1 Vietnam Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.4.10.2 Vietnam Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.4.10.3 Vietnam Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.4.11 Singapore
10.4.11.1 Singapore Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.4.11.2 Singapore Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.4.11.3 Singapore Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.4.12 Australia
10.4.12.1 Australia Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.4.12.2 Australia Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.4.12.3 Australia Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.4.13 Rest of Asia Pacific
10.4.13.1 Rest of Asia Pacific Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.4.13.2 Rest of Asia Pacific Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.4.13.3 Rest of Asia Pacific Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.5 Middle East and Africa
10.5.1 Middle East
10.5.1.1 Trends Analysis
10.5.1.2 Middle East Gene Therapy Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.5.1.3 Middle East Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.5.1.4 Middle East Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.5.1.5 Middle East Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.5.1.6 UAE
10.5.1.6.1 UAE Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.5.1.6.2 UAE Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.5.1.6.3 UAE Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.5.1.7 Egypt
10.5.1.7.1 Egypt Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.5.1.7.2 Egypt Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.5.1.7.3 Egypt Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.5.1.8 Saudi Arabia
10.5.1.8.1 Saudi Arabia Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.5.1.8.2 Saudi Arabia Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.5.1.8.3 Saudi Arabia Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.5.1.9 Qatar
10.5.1.9.1 Qatar Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.5.1.9.2 Qatar Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.5.1.9.3 Qatar Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.5.1.10 Rest of Middle East
10.5.1.10.1 Rest of Middle East Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.5.1.10.2 Rest of Middle East Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.5.1.10.3 Rest of Middle East Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.5.2 Africa
10.5.2.1 Trends Analysis
10.5.2.2 Africa Gene Therapy Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.5.2.3 Africa Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.5.2.4 Africa Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.5.2.5 Africa Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.5.2.6 South Africa
10.5.2.6.1 South Africa Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.5.2.6.2 South Africa Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.5.2.6.3 South Africa Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.5.2.7 Nigeria
10.5.2.7.1 Nigeria Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.5.2.7.2 Nigeria Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.5.2.7.3 Nigeria Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.5.2.8 Rest of Africa
10.5.2.8.1 Rest of Africa Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.5.2.8.2 Rest of Africa Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.5.2.8.3 Rest of Africa Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.6 Latin America
10.6.1 Trends Analysis
10.6.2 Latin America Gene Therapy Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.6.3 Latin America Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.6.4 Latin America Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.6.5 Latin America Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.6.6 Brazil
10.6.6.1 Brazil Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.6.6.2 Brazil Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.6.6.3 Brazil Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.6.7 Argentina
10.6.7.1 Argentina Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.6.7.2 Argentina Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.6.7.3 Argentina Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.6.8 Colombia
10.6.8.1 Colombia Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.6.8.2 Colombia Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.6.8.3 Colombia Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
10.6.9 Rest of Latin America
10.6.9.1 Rest of Latin America Gene Therapy Market Estimates and Forecasts, by Indication (2020-2032) (USD Billion)
10.6.9.2 Rest of Latin America Gene Therapy Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.6.9.3 Rest of Latin America Gene Therapy Market Estimates and Forecasts, by Vector Type (2020-2032) (USD Billion)
11. Company Profiles
11.1 BioMarin
11.1.1 Company Overview
11.1.2 Financial
11.1.3 Indication s/ Indication s Offered
11.1.4 SWOT Analysis
11.2 Amgen Inc.
11.2.1 Company Overview
11.2.2 Financial
11.2.3 Indication s/ Indication s Offered
11.2.4 SWOT Analysis
11.3 F. Hoffmann-La Roche
11.3.1 Company Overview
11.3.2 Financial
11.3.3 Indication s/ Indication s Offered
11.3.4 SWOT Analysis
11.4 Gilead Sciences, Inc.
11.4.1 Company Overview
11.4.2 Financial
11.4.3 Indication s/ Indication s Offered
11.4.4 SWOT Analysis
11.5 Merck & Co.
11.5.1 Company Overview
11.5.2 Financial
11.5.3 Indication s/ Indication s Offered
11.5.4 SWOT Analysis
11.6 Legend Biotech
11.6.1 Company Overview
11.6.2 Financial
11.6.3 Indication s/ Indication s Offered
11.6.4 SWOT Analysis
11.7 Bristol-Myers Squibb Company
11.7.1 Company Overview
11.7.2 Financial
11.7.3 Indication s/ Indication s Offered
11.7.4 SWOT Analysis
11.8 Sarepta Therapeutics, Inc.
11.8.1 Company Overview
11.8.2 Financial
11.8.3 Indication s/ Indication s Offered
11.8.4 SWOT Analysis
11.9 Novartis AG
11.9.1 Company Overview
11.9.2 Financial
11.9.3 Indication s/ Indication s Offered
11.9.4 SWOT Analysis
11.10 uniQure N.V.
11.10.1 Company Overview
11.10.2 Financial
11.10.3 Indication s/ Indication s Offered
11.10.4 SWOT Analysis
12. Use Cases and Best Practices
13. Conclusion
An accurate research report requires proper strategizing as well as implementation. There are multiple factors involved in the completion of good and accurate research report and selecting the best methodology to compete the research is the toughest part. Since the research reports we provide play a crucial role in any company’s decision-making process, therefore we at SNS Insider always believe that we should choose the best method which gives us results closer to reality. This allows us to reach at a stage wherein we can provide our clients best and accurate investment to output ratio.
Each report that we prepare takes a timeframe of 350-400 business hours for production. Starting from the selection of titles through a couple of in-depth brain storming session to the final QC process before uploading our titles on our website we dedicate around 350 working hours. The titles are selected based on their current market cap and the foreseen CAGR and growth.
The 5 steps process:
Step 1: Secondary Research:
Secondary Research or Desk Research is as the name suggests is a research process wherein, we collect data through the readily available information. In this process we use various paid and unpaid databases which our team has access to and gather data through the same. This includes examining of listed companies’ annual reports, Journals, SEC filling etc. Apart from this our team has access to various associations across the globe across different industries. Lastly, we have exchange relationships with various university as well as individual libraries.

Step 2: Primary Research
When we talk about primary research, it is a type of study in which the researchers collect relevant data samples directly, rather than relying on previously collected data. This type of research is focused on gaining content specific facts that can be sued to solve specific problems. Since the collected data is fresh and first hand therefore it makes the study more accurate and genuine.
We at SNS Insider have divided Primary Research into 2 parts.
Part 1 wherein we interview the KOLs of major players as well as the upcoming ones across various geographic regions. This allows us to have their view over the market scenario and acts as an important tool to come closer to the accurate market numbers. As many as 45 paid and unpaid primary interviews are taken from both the demand and supply side of the industry to make sure we land at an accurate judgement and analysis of the market.
This step involves the triangulation of data wherein our team analyses the interview transcripts, online survey responses and observation of on filed participants. The below mentioned chart should give a better understanding of the part 1 of the primary interview.

Part 2: In this part of primary research the data collected via secondary research and the part 1 of the primary research is validated with the interviews from individual consultants and subject matter experts.
Consultants are those set of people who have at least 12 years of experience and expertise within the industry whereas Subject Matter Experts are those with at least 15 years of experience behind their back within the same space. The data with the help of two main processes i.e., FGDs (Focused Group Discussions) and IDs (Individual Discussions). This gives us a 3rd party nonbiased primary view of the market scenario making it a more dependable one while collation of the data pointers.
Step 3: Data Bank Validation
Once all the information is collected via primary and secondary sources, we run that information for data validation. At our intelligence centre our research heads track a lot of information related to the market which includes the quarterly reports, the daily stock prices, and other relevant information. Our data bank server gets updated every fortnight and that is how the information which we collected using our primary and secondary information is revalidated in real time.

Step 4: QA/QC Process
After all the data collection and validation our team does a final level of quality check and quality assurance to get rid of any unwanted or undesired mistakes. This might include but not limited to getting rid of the any typos, duplication of numbers or missing of any important information. The people involved in this process include technical content writers, research heads and graphics people. Once this process is completed the title gets uploader on our platform for our clients to read it.
Step 5: Final QC/QA Process:
This is the last process and comes when the client has ordered the study. In this process a final QA/QC is done before the study is emailed to the client. Since we believe in giving our clients a good experience of our research studies, therefore, to make sure that we do not lack at our end in any way humanly possible we do a final round of quality check and then dispatch the study to the client.
Key Segments
By Indication
Acute Lymphoblastic Leukemia (ALL)
Inherited Retinal Disease
Large B-Cell Lymphoma
Melanoma (lesions)
Beta-Thalassemia Major/SCD
Spinal Muscular Atrophy (SMA)
Others
By Route of Administration
Intravenous
Others
By Vector Type
Lentivirus
AAV
RetroVirus & gamma RetroVirus
Modified Herpes Simplex Virus
Adenovirus
Others
Request for Segment Customization as per your Business Requirement: Segment Customization Request
Regional Coverage:
North America
US
Canada
Mexico
Europe
Eastern Europe
Poland
Romania
Hungary
Turkey
Rest of Eastern Europe
Western Europe
Germany
France
UK
Italy
Spain
Netherlands
Switzerland
Austria
Rest of Western Europe
Asia Pacific
China
India
Japan
South Korea
Vietnam
Singapore
Australia
Rest of Asia Pacific
Middle East & Africa
Middle East
UAE
Egypt
Saudi Arabia
Qatar
Rest of the Middle East
Africa
Nigeria
South Africa
Rest of Africa
Latin America
Brazil
Argentina
Colombia
Rest of Latin America
Request for Country Level Research Report: Country Level Customization Request
Available Customization
With the given market data, SNS Insider offers customization as per the company’s specific needs. The following customization options are available for the report:
Product Analysis
Criss-Cross segment analysis (e.g. Product X Application)
Product Matrix which gives a detailed comparison of the product portfolio of each company
Geographic Analysis
Additional countries in any of the regions
Company Information
Detailed analysis and profiling of additional market players (Up to five)
Dental Lasers Market was estimated at USD 339.95 million in 2023 and is expected to reach USD 664.60 million by 2032, growing at a CAGR of 7.76% from 2024-2032.
The Cell Signaling Market Size was valued at USD 5.1 billion in 2023 and is expected to reach USD 9.4 billion by 2032 and grow at a CAGR of 7.1% over the forecast period 2024-2032.
Immunoassay Analyzers Market was valued at USD 7.55 billion in 2023, expected to reach USD 10.16 billion by 2032, growing at a CAGR of 3.38% from 2024-2032.
The Tissue Diagnostics Market Size was valued at USD 6.83 billion in 2023 and is expected to reach USD 14.18 billion by 2032 and grow at a CAGR of 8.47% over the forecast period 2024-2032.
The Intraoperative Imaging Market Size was valued at USD 2.9 Billion in 2023 and is expected to reach USD 4.85 Billion by 2032, growing at a CAGR of 5.9% over the forecast period 2024-2032.
The Global Biobanking Market Size, valued at USD 76.18 Billion in 2023, is expected to grow to USD 160.75 Billion by 2032, with a CAGR of 8.67%.
Hi! Click one of our member below to chat on Phone