The Electronic Trial Master File (eTMF) Systems Market Size was valued at USD 1.68 billion in 2023, and is expected to reach USD 4.40 billion by 2031 and grow at a CAGR of 12.8% over the forecast period 2024-2031.
The main electronic experimental file (containing digital content) is known as "eTMF." It is a type of pharmaceutical business content management system, which provides a systematic way of organizing and storing papers, photographs, and other digital content for clinical trials that may be required to comply with government regulatory agencies. Throughout the life cycle of controlled clinical research content, a number of techniques, methods, and techniques are used, all of which are listed under the term "eTMF." Controlled clinical trial content can be managed using the eTMF system, which includes hardware and software.
Get more information on Electronic Trial Master File (eTMF) Systems Market - Request Sample Report
The rising usage of electronic trial master file market systems is primarily responsible for the growth of the worldwide electronic trial master file market. Additionally, there have been more clinical trials conducted as well as partnerships between contract research organisations and biopharmaceutical firms expanding, all of which are anticipated to support the growth of the global electronic trial master file market over the course of the assessment period. Additionally, enhanced clinical trials and R&D efforts supported by biotech and pharmaceutical firms are anticipated to fuel market expansion during the assessment period.
DRIVERS
Growing Use of eTMF Systems
Upsurge in the Number of Clinical Trials
Increasing Government Support and Grants for Clinical Trials
R&D Spending Growth by Pharma-Biotech Companies and Its Expenditure Allocations
RESTRAINTS
Budgetary restrictions
Escalating worries about the privacy and security of clinical data.
Lack of knowledgeable and talented professionals
The regulatory environment is always changing.
OPPORTUNITIES
Expansion of clinical trial numbers
New Asian Markets
CHALLENGES
Patient Data Privacy
Lack of Skilled Personnel to Operate eTMF Systems
The eTMF Systems Market has seen significant shift as a result of Covid 19. In order to reduce the reliance on manual processes, it has become more important than ever to incorporate digital technologies into clinical activities. The epidemic has provided a chance to swiftly and creatively capture and maintain clinical trial documents. Organizations have turned to the eTMF systems idea to integrate digitalization while handling clinical data in order to take advantage of the new opportunities in the post-Covid era.
By Delivery Mode
On-premise and cloud-based eTMF are two market segments. The market's largest share belonged to the cloud-based eTMF category. This delivery mode's flexibility, scalability, and affordability are principally responsible for its sizable market share.
By Component
Services and software make up the market segments. The largest market share was held by the services sector. Because of their recurring needs and vital nature, this category accounts for a sizable portion of the market. For consultation, data storage, deploying services, training, maintenance, and routine upgrades of products, end-users of eTMF systems rely largely on service providers.
By End-User
Contract research organisations (CROs), pharmaceutical and biotechnology corporations, and other end-users are the market segments for eTMF systems (medical device companies, academic research institutes, and consulting service companies). The segment of pharmaceutical and biotechnology firms held the biggest market share. The use of eTMF systems in this end-user segment will be fueled by the growing number of clinical project management applications for the software and the presence of sizable R&D budgets with major pharmaceutical and biotechnology businesses.
By Delivery Mode
Cloud-based eTMF
On-premise eTMF
By Component
Services
Software
By End-User
Pharmaceutical & biotechnology companies
Contract Research Organizations (CROs)
Other end-users
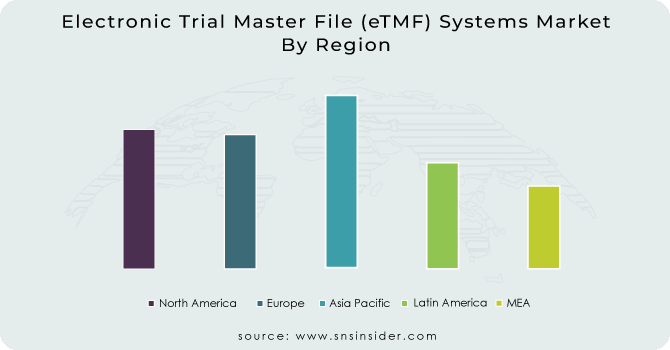
The greatest regional region that contributes to the market's expansion on a worldwide scale is the Americas. Oracle and Veeva Systems are two prominent companies that account for a sizable share of the market value for Electronic Trial Master File (eTMF) Systems. At the international level, the European market has been exhibiting strong performance. The strong backing from regulatory bodies is one of the primary forces that influence the market.
The adoption of quickly developing technology in the market is one of the key Electronic Trial Master File (eTMF) Systems Market Trends that is consistent across various regional sectors. The Asia Pacific area is anticipated to display strong market performance throughout the anticipated timeframe. Geographically speaking, the Middle East and Africa make up the smallest segment. The inadequate advancement of the region's healthcare infrastructure and the lack of exposure to new digital technologies and innovations are the primary causes of the eTMF Systems Market's slow growth. Each geographical division is predicted to contribute to the success of the worldwide market during the anticipated time frame.
Need any customization research on Electronic Trial Master File (eTMF) Systems Market - Enquiry Now
REGIONAL COVERAGE:
North America
USA
Canada
Mexico
Europe
Germany
UK
France
Italy
Spain
The Netherlands
Rest of Europe
Asia-Pacific
Japan
south Korea
China
India
Australia
Rest of Asia-Pacific
The Middle East & Africa
Israel
UAE
South Africa
Rest of Middle East & Africa
Latin America
Brazil
Argentina
Rest of Latin America
Some of the major key players of Electronic Trial Master File (eTMF) Systems Market are as follows: Aurea, Inc., Master Control Inc., Covance Inc., Ennov, Oracle, Veeva Systems, Care Lex, Trans perfect, ePharma Solutions, SureClinical Inc., Phlex global, Database Integrations, Inc. and Other Players.

| Report Attributes | Details |
|---|---|
| Market Size in 2023 | US$ 1.68 Billion |
| Market Size by 2031 | US$ 4.40 Billion |
| CAGR | CAGR of 12.8% From 2024 to 2031 |
| Base Year | 2023 |
| Forecast Period | 2024-2031 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Delivery Mode (Cloud-based eTMF, On-premise eTMF) • By Component (Services, Software) • By End-User (Pharmaceutical & biotechnology companies, Contract Research Organizations (CROs), Other end-users) |
| Regional Analysis/Coverage | North America (USA, Canada, Mexico), Europe (Germany, UK, France, Italy, Spain, Netherlands, Rest of Europe), Asia-Pacific (Japan, South Korea, China, India, Australia, Rest of Asia-Pacific), The Middle East & Africa (Israel, UAE, South Africa, Rest of Middle East & Africa), Latin America (Brazil, Argentina, Rest of Latin America) |
| Company Profiles | Aurea, Inc., Master control Inc., Covance Inc., Ennov, Oracle, Veeva Systems, Care Lex, Trans perfect, ePharma Solutions, SureClinical Inc., Phlex global, Database Integrations, Inc. |
| DRIVERS | • Growing Use of eTMF Systems • Upsurge in the Number of Clinical Trials • Increasing Government Support and Grants for Clinical Trials |
| RESTRAINTS | • Budgetary restrictions • Escalating worries about the privacy and security of clinical data. • Lack of knowledgeable and talented professionals |
Ans: USD 1.68 billion is the market value in 2023.
Ans: The CAGR of the Electronic Trial Master File (ETMF) Systems Market is 12.8%.
Ans: The North America region high share of the Electronic Trial Master File (ETMF) Systems Market
Expansion of clinical trial numbers
New Asian Markets
Ans: The forecast period of the Electronic Trial Master File (ETMF) Systems Market is 2024-2031.
Table of Contents
1. Introduction
1.1 Market Definition
1.2 Scope
1.3 Research Assumptions
2. Research Methodology
3. Market Dynamics
3.1 Drivers
3.2 Restraints
3.3 Opportunities
3.4 Challenges
4. Impact Analysis
4.1 COVID-19 Impact Analysis
4.2 Impact of Ukraine- Russia War
4.3 Impact of Ongoing Recession
4.3.1 Introduction
4.3.2 Impact on major economies
4.3.2.1 US
4.3.2.2 Canada
4.3.2.3 Germany
4.3.2.4 France
4.3.2.5 United Kingdom
4.3.2.6 China
4.3.2.7 Japan
4.3.2.8 South Korea
4.3.2.9 Rest of the World
5. Value Chain Analysis
6. Porter’s 5 forces model
7. PEST Analysis
8. Electronic Trial Master File (eTMF) Systems Market Segmentation, By Delivery Mode
8.1 Cloud-based eTMF
8.2 On-premise eTMF
9. Electronic Trial Master File (eTMF) Systems Market Segmentation, By Component
9.1 Services
9.2 Software
10. Electronic Trial Master File (eTMF) Systems Market Segmentation, By End-User
10.1 Pharmaceutical & biotechnology companies
10.2 Contract Research Organizations (CROs)
10.3 Other end-users
11. Regional Analysis
11.1 Introduction
11.2 North America
11.2.1 USA
11.2.2 Canada
11.2.3 Mexico
11.3 Europe
11.3.1 Germany
11.3.2 UK
11.3.3 France
11.3.4 Italy
11.3.5 Spain
11.3.6 The Netherlands
11.3.7 Rest of Europe
11.4 Asia-Pacific
11.4.1 Japan
11.4.2 South Korea
11.4.3 China
11.4.4 India
11.4.5 Australia
11.4.6 Rest of Asia-Pacific
11.5 The Middle East & Africa
11.5.1 Israel
11.5.2 UAE
11.5.3 South Africa
11.5.4 Rest
11.6 Latin America
11.6.1 Brazil
11.6.2 Argentina
11.6.3 Rest of Latin America
12. Company Profiles
12.1 Aurea, Inc.
12.1.1 Financial
12.1.2 Products/ Services Offered
12.1.3 SWOT Analysis
12.1.4 The SNS view
12.2 Master control Inc.
12.3 Covance Inc.
12.4 Ennov
12.5 Oracle
12.6 Veeva Systems
12.7 Care Lex
12.8 Trans perfect
12.9 ePharma Solutions
12.10 SureClinical Inc.
13. Competitive Landscape
13.1 Competitive Benchmark
13.2 Market Share Analysis
13.3 Recent Developments
14. Conclusion
An accurate research report requires proper strategizing as well as implementation. There are multiple factors involved in the completion of good and accurate research report and selecting the best methodology to compete the research is the toughest part. Since the research reports we provide play a crucial role in any company’s decision-making process, therefore we at SNS Insider always believe that we should choose the best method which gives us results closer to reality. This allows us to reach at a stage wherein we can provide our clients best and accurate investment to output ratio.
Each report that we prepare takes a timeframe of 350-400 business hours for production. Starting from the selection of titles through a couple of in-depth brain storming session to the final QC process before uploading our titles on our website we dedicate around 350 working hours. The titles are selected based on their current market cap and the foreseen CAGR and growth.
The 5 steps process:
Step 1: Secondary Research:
Secondary Research or Desk Research is as the name suggests is a research process wherein, we collect data through the readily available information. In this process we use various paid and unpaid databases which our team has access to and gather data through the same. This includes examining of listed companies’ annual reports, Journals, SEC filling etc. Apart from this our team has access to various associations across the globe across different industries. Lastly, we have exchange relationships with various university as well as individual libraries.

Step 2: Primary Research
When we talk about primary research, it is a type of study in which the researchers collect relevant data samples directly, rather than relying on previously collected data. This type of research is focused on gaining content specific facts that can be sued to solve specific problems. Since the collected data is fresh and first hand therefore it makes the study more accurate and genuine.
We at SNS Insider have divided Primary Research into 2 parts.
Part 1 wherein we interview the KOLs of major players as well as the upcoming ones across various geographic regions. This allows us to have their view over the market scenario and acts as an important tool to come closer to the accurate market numbers. As many as 45 paid and unpaid primary interviews are taken from both the demand and supply side of the industry to make sure we land at an accurate judgement and analysis of the market.
This step involves the triangulation of data wherein our team analyses the interview transcripts, online survey responses and observation of on filed participants. The below mentioned chart should give a better understanding of the part 1 of the primary interview.

Part 2: In this part of primary research the data collected via secondary research and the part 1 of the primary research is validated with the interviews from individual consultants and subject matter experts.
Consultants are those set of people who have at least 12 years of experience and expertise within the industry whereas Subject Matter Experts are those with at least 15 years of experience behind their back within the same space. The data with the help of two main processes i.e., FGDs (Focused Group Discussions) and IDs (Individual Discussions). This gives us a 3rd party nonbiased primary view of the market scenario making it a more dependable one while collation of the data pointers.
Step 3: Data Bank Validation
Once all the information is collected via primary and secondary sources, we run that information for data validation. At our intelligence centre our research heads track a lot of information related to the market which includes the quarterly reports, the daily stock prices, and other relevant information. Our data bank server gets updated every fortnight and that is how the information which we collected using our primary and secondary information is revalidated in real time.

Step 4: QA/QC Process
After all the data collection and validation our team does a final level of quality check and quality assurance to get rid of any unwanted or undesired mistakes. This might include but not limited to getting rid of the any typos, duplication of numbers or missing of any important information. The people involved in this process include technical content writers, research heads and graphics people. Once this process is completed the title gets uploader on our platform for our clients to read it.
Step 5: Final QC/QA Process:
This is the last process and comes when the client has ordered the study. In this process a final QA/QC is done before the study is emailed to the client. Since we believe in giving our clients a good experience of our research studies, therefore, to make sure that we do not lack at our end in any way humanly possible we do a final round of quality check and then dispatch the study to the client.
The Clinical Documentation Improvement Market Size was assessed to be worth USD 5.13 billion in 2023 and is expected to increase to USD 9.96 billion by 2032.
The Digital PCR Market size was valued at USD 6.77 billion in 2023 & projected to reach USD 14.89 billion by 2032, at a CAGR of 9.16% by 2024-2032.
The Neurorehabilitation Market was valued at USD 1.95 billion in 2023 and expected to reach USD 6.18 billion by 2032, at a CAGR of 13.68%.
The Monkeypox Testing Market Size was valued at USD 1.73 billion in 2023 and is expected to reach USD 2.58 billion by 2032 and grow at a CAGR of 4.55% over the forecast period 2024-2032.
The Opioid Use Disorder Market Size was valued at USD 3.7 Billion in 2023 and is expected to reach USD 9.02 Billion by 2032, growing at a CAGR of 10.4%.
The Leptospirosis Market was valued at USD 501.0 million in 2023 and is expected to reach USD 868.2 million by 2032 with a growing CAGR of 6.3% from 2024 to 2032.
Hi! Click one of our member below to chat on Phone