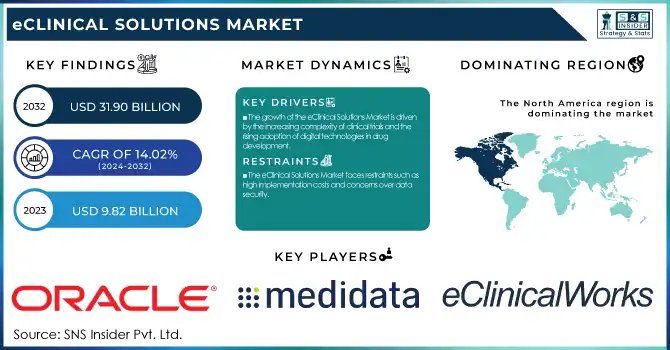
The Global eClinical Solutions Market was valued at USD 9.82 Billion in 2023 and is projected to reach USD 31.90 Billion by 2032, growing at a 14.02% CAGR over the forecast period 2024-2032.
Get more information on eClinical Solutions Market - Request Free Sample Report
This report emphasizes the growth in the adoption of eClinical solutions in regions due to the requirement for efficient clinical trial management and regulatory compliance. The research analyzes the growing number of clinical trials leveraging eClinical technologies, with an emphasis on regional differences and how digital transformation in clinical research is influencing this. The report also discusses the growth in investment and funding, with stakeholders acknowledging the efficiency and cost-effectiveness of such solutions. The study also explores pharmaceutical and healthcare expenditures on eClinical solutions, capturing the increasing focus on data-driven decision-making, remote monitoring, and AI-powered analytics in clinical trials.
Drivers
The growth of the eClinical Solutions Market is driven by the increasing complexity of clinical trials and the rising adoption of digital technologies in drug development.
The growth of decentralized and hybrid clinical trials has driven the demand for electronic data capture (EDC), remote patient monitoring, and eCOA solutions. Regulatory agencies like the FDA and EMA are actively encouraging the use of electronic systems to improve data integrity and trial efficiency. Moreover, the rise in R&D expenditure by biotech and pharma companies, which amounted to more than USD 230 billion across the world in 2023, has pushed the demand for strong clinical trial management solutions further. The inclusion of AI and machine learning into eClinical platforms is also enhancing patient recruitment, trial monitoring, and risk-based monitoring strategies. In addition, collaborations between CROs and eClinical solution providers, like the one between Medidata and Labcorp, have improved operational efficiencies in clinical research. The increasing amount of clinical trial data has also created a need for advanced analytics platforms, further driving the market.
Restraints
The eClinical Solutions Market faces restraints such as high implementation costs and concerns over data security.
The upfront cost involved in implementing advanced eClinical solutions, such as EDC and CTMS, can be high, constraining adoption among small and mid-tier pharmaceutical companies and academic research institutions. Regulatory compliance issues across regions also make adoption of uniform eClinical platforms complex. Cybersecurity threats are another major obstacle, with healthcare data breaches increasing more than 50% in 2023, raising fears regarding patient confidentiality and data integrity. A significant constraint is also resistance to change among established clinical research organizations where paper-based environments continue to operate due to familiarity and perceived stability. Inadequate interoperability between various eClinical platforms and legacy systems further impedes integrated data handling. Furthermore, the lack of highly qualified professionals skilled enough to operate complex eClinical software solutions creates an operational burden for most research organizations.
Opportunities
The eClinical Solutions Market presents significant opportunities with the increasing shift towards artificial intelligence (AI) and big data analytics in clinical research.
AI-powered eClinical systems are enhancing patient recruitment effectiveness through the analysis of large datasets to determine suitable candidates, lowering dropout rates in trials. Moreover, the increasing use of blockchain technology in clinical trials provides better data security, transparency, and tamper-proof documentation, which caters to the needs of regulatory bodies. Expansion of eClinical solutions in developing markets is also a significant opportunity, as clinical trial activity is increasing in such markets due to reduced operational costs and heterogeneous patient populations. Increased adoption of wearable devices and remote monitoring instruments in clinical trials also offers a profitable avenue for eClinical solution providers, enabling real-time data capture and enhanced patient compliance. In addition, regulatory bodies are promoting digitalization, with the FDA's Real-World Evidence (RWE) Program urging increased application of digital solutions in clinical research. The growth in cloud-based eClinical solutions, which had more than 90% of deployments in 2023, also offers scalability and affordable alternatives for research organizations. Strategic partnerships between technology companies and CROs, including the Veeva Systems tie-up with several pharmaceutical majors, are likely to unlock more opportunities in this segment.
Challenges
The eClinical Solutions Market faces challenges in achieving widespread interoperability and standardization across clinical research organizations.
Most eClinical platforms use various architectures, which does not allow integration of the data seamlessly, creating inefficiencies in trial management. Regulatory compliance is also not the same in all regions, and companies need to keep updating their platforms to comply with emerging requirements, which raises operating expenses. Another major challenge is excessive dependence on internet connectivity for cloud-based solutions, especially in geographically remote regions where clinical trials are now being implemented. Legacy system data migration to contemporary eClinical solutions is also a complicated task, tending to result in data loss or inconsistencies if not properly handled. Additionally, patient participation in digital trials is still a challenge since not all patients are willing to use technology-based solutions like ePRO and eConsent, resulting in potential data gaps. The absence of harmonized AI regulations for clinical trials also raises uncertainty about how much decision-making by AI can be adopted. Moreover, pushback from clinical trial professionals who are not accustomed to AI and big data analytics hinders uptake. The market further grapples with escalating cybersecurity risks, with cyberattacks in the healthcare sector increasing by 40% in 2023, which calls for more robust data protection practices for eClinical platforms.
By Product
The Clinical Trial Management Systems (CTMS) segment led the eClinical Solutions Market in 2023 with a 22.2% share of the total revenue. The leadership of CTMS is due to its pivotal position in managing clinical trial operations, enhancing study efficiency, and maintaining regulatory compliance. The increasing volume of complex multi-site trials and the need for real-time tracking of trials have also fueled the use of CTMS solutions.
The Electronic Clinical Outcome Assessment (eCOA) market is expected to be the fastest-growing during the forecast period. The reasons behind this are rising interest in patient-centric trials, demand for real-world evidence, and favorable regulations towards digital health technologies. Decentralized and hybrid trials have also created an additional momentum in demand for eCOA solutions, as these solutions drive greater patient engagement and data accuracy.
By Delivery Mode
The Web and Cloud-based business segment led the market with a strong 93.3% revenue share in 2023. The sheer popularity of cloud-based solutions lies in their flexibility, affordability, and capacity for remote clinical trials. Furthermore, the need for smooth data consolidation and real-time access to trial data has accelerated the adoption of web-based eClinical platforms.
The On-Premise segment, though having a smaller market share, is anticipated to be the highest-growing segment over the forecast period. This is primarily because of data security and compliance issues in highly regulated industries, which make some organizations prefer on-premise solutions for more control over clinical trial data.
By Development Phases
The Phase III segment occupied the maximum share of 51.3% in 2023, due to the complexity and high costs involved in conducting late-stage clinical trials. Phase III trials include large patient groups and extensive data collection, and hence eClinical solution adoption becomes critical for achieving maximum trial efficiency and for regulatory submission purposes. The growth in drug approvals and biopharmaceutical R&D spend have also enhanced the demand for eClinical solutions in the Phase III segment.
The Phase I segment is anticipated to be the fastest-growing during the forecast period. This is spurred by the growth in early-stage clinical trials, the adoption of adaptive trial designs, and the growing focus on AI-based patient recruitment strategies. The need for data-driven decision-making in early-phase trials is driving the adoption of eClinical solutions in this segment.
By End-use
The Contract Research Organizations (CROs) segment was the market leader, with 37.5% of the overall revenue in 2023. The trend of outsourcing clinical trials to CROs has been a key driver of this leadership. CROs are utilizing eClinical solutions to increase trial efficiency, improve regulatory compliance, and shorten drug development timelines for pharmaceutical and biotechnology firms. The complexity of clinical trials is also driving demand for technology-enabled CRO services.
The Pharma and Biotech Organizations segment is projected to experience major growth over the coming years. The escalation in R&D expenditures, biopharmaceutical pipelines expansion, and implementation of digital technologies to be used for clinical development are significant drivers propelling the segment growth. Further, the need to implement decentralized clinical trials and analytics through artificial intelligence is expanding pharmaceutical and biotech companies' requirements for cutting-edge eClinical solutions.
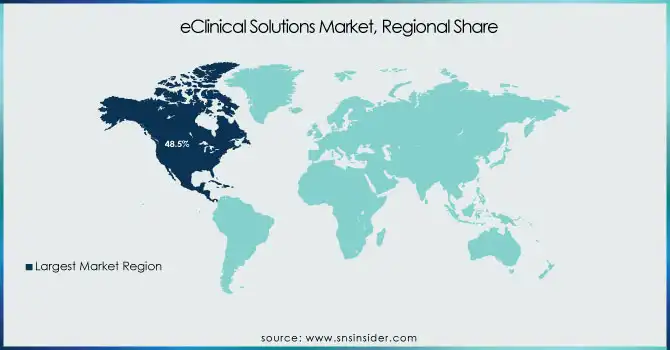
North America held the largest share in the eClinical Solutions Market in 2023, holding 48.5% of the overall revenue share. Its leadership is propelled by the predominant presence of pharma and biotech firms, widespread use of cutting-edge clinical trial technologies, and favorable regulatory environments. The U.S., in turn, is a leading contributor with high R&D spending, rising numbers of clinical trials, and rising demands for decentralized trial solutions. Also, the availability of key players and regular strategic partnerships among technology providers, CROs, and pharmaceutical firms further enhance North America's leadership. The heavy adoption of cloud-based eClinical solutions, combined with government efforts to develop clinical research infrastructure, has continued to drive market growth in the region.
Asia-Pacific is expected to be the most rapidly growing region during the forecast period. The growing number of clinical trials, greater investments from international pharmaceutical organizations, and cost benefits in trial conduct in countries such as China, India, and Japan are major drivers of growth. Further, the region is also experiencing quick digitalization of healthcare, as well as regulatory reforms that favor the implementation of eClinical solutions. The growing emphasis on precision medicine and analytics driven by artificial intelligence is also fueling market growth in Asia-Pacific, making it a leading growth center for the eClinical solutions market.
Need any customization research on eClinical Solutions Market - Enquiry Now
Fountayn (formerly Datatrak International, Inc.) – Unified eClinical Platform
Oracle – Oracle Health Sciences Clinical One
Calyx (formerly part of Parexel International Corporation) – Calyx CTMS, Calyx EDC, Calyx IRT
Medidata (Dassault Systèmes) – Medidata Rave EDC, Medidata Rave CTMS, Medidata eCOA, Medidata RTSM
CRF Health (Signant Health) – Signant SmartSignals eCOA, Signant SmartSignals EDC
Clario (ERT and Bioclinica) – Clario eCOA, Clario EDC, Clario CTMS, Clario RTSM
eClinicalWorks – eClinicalWorks EHR & PM Solutions (includes clinical trial management features)
Merative (IBM Watson Health) – Merative Clinical Development
Anju Software – Anju eClinical Suite, Anju CTMS, Anju EDC
eClinical Solutions – elluminate Clinical Data Cloud
MaxisIT – MaxisIT Integrated Clinical Trial Data Platform
IQVIA – IQVIA Orchestrated Clinical Trials Suite, IQVIA EDC, IQVIA CTMS
Castor – Castor EDC, Castor eConsent, Castor ePRO
Veeva Systems – Veeva Vault EDC, Veeva Vault CTMS, Veeva Vault eTMF
Recent Developments
In Feb 2025, eClinical Solutions LLC strengthened its market position in 2024, adding 20 new clients, including three CROs, and serving 16 of the top 50 pharmaceutical companies. To enhance clinical trial efficiency, the company introduced AI-driven innovations, including a GenAI-powered chatbot and Clean Progress Tracking, aimed at reducing cycle times and improving operational performance.
In Dec 2024, eClinical Solutions partnered with Snowflake to integrate its elluminate Clinical Data Cloud with the Snowflake AI Data Cloud, enabling life sciences organizations to optimize clinical data management. This collaboration enhances AI-driven analytics and streamlines data architecture for improved healthcare outcomes.
| Report Attributes | Details |
| Market Size in 2023 | USD 9.82 billion |
| Market Size by 2032 | USD 31.90 billion |
| CAGR | CAGR of 14.02% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Product [Electronic Data Capture (EDC) and Clinical Data Management Systems (CDMS), Clinical Trial Management Systems (CTMS), Clinical Analytics Platforms, Randomization and Trial Supply Management (RTSM), Clinical Data Integration Platforms, Electronic Clinical Outcome Assessment (eCOA), Safety Solutions, Electronic Trial Master File (eTMF), Electronic Consent (eConsent)] • By Delivery Mode [Web and Cloud based, On- Premise] • By Development Phases [Phase I, Phase II, Phase III, Phase IV] • By End-use [Hospitals/Healthcare providers, CROs, Academic Institutes, Pharma & Biotech Organizations, Medical Device Manufacturers] |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | Fountayn, Oracle, Calyx, Medidata, CRF Health, Clario, eClinicalWorks, Merative, Anju Software, eClinical Solutions, MaxisIT, IQVIA, Castor, Veeva Systems. |
Top-down, bottom-up, Quantitative, Qualitative Research, Descriptive, Analytical, Applied, Fundamental Research.
Licensed Enterprise (On-premise), Web-hosted (On-demand), Cloud-based (SaaS) are the sub segments of by Mode of Delivery.
Key drivers of the eClinical Solutions Market the demand for low-cost drug discovery and development is growing, and Supporting Regenerative Medicine Research with Public and Private Funding.
Segments of eClinical Solutions market are By Product, By Mode of Delivery, By Clinical Trial Phase, and By End User.
The eClinical Solutions Market Size was valued at USD 9.82 Billion in 2023 and is expected to reach USD 31.90 Billion by 2032 and grow at a CAGR of 14.02% over the forecast period 2024-2032.
Table Of Contents:
1. Introduction
1.1 Market Definition
1.2 Scope (Inclusion and Exclusions)
1.3 Research Assumptions
2. Executive Summary
2.1 Market Overview
2.2 Regional Synopsis
2.3 Competitive Summary
3. Research Methodology
3.1 Top-Down Approach
3.2 Bottom-up Approach
3.3. Data Validation
3.4 Primary Interviews
4. Market Dynamics Impact Analysis
4.1 Market Driving Factors Analysis
4.1.1 Drivers
4.1.2 Restraints
4.1.3 Opportunities
4.1.4 Challenges
4.2 PESTLE Analysis
4.3 Porter’s Five Forces Model
5. Statistical Insights and Trends Reporting
5.1 Adoption Trends of eClinical Solutions, by Region (2023)
5.2 Clinical Trials Managed with eClinical Solutions, by Region (2020-2032)
5.3 Investment and Funding in eClinical Solutions (2023)
5.4 Healthcare and Pharmaceutical Spending on eClinical Solutions (2023)
6. Competitive Landscape
6.1 List of Major Companies, By Region
6.2 Market Share Analysis, By Region
6.3 Product Benchmarking
6.3.1 Product specifications and features
6.3.2 Pricing
6.4 Strategic Initiatives
6.4.1 Marketing and Promotional Activities
6.4.2 Distribution and Supply Chain Strategies
6.4.3 Expansion plans and new product launches
6.4.4 Strategic partnerships and collaborations
6.5 Technological Advancements
6.6 Market Positioning and Branding
7. eClinical Solutions Market Segmentation, by Product
7.1 Chapter Overview
7.2 Electronic Data Capture (EDC) and Clinical Data Management Systems (CDMS)
7.2.1 Electronic Data Capture (EDC) and Clinical Data Management Systems (CDMS) Market Trends Analysis (2020-2032)
7.2.2 Electronic Data Capture (EDC) and Clinical Data Management Systems (CDMS) Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3 Clinical Trial Management Systems (CTMS)
7.3.1 Clinical Trial Management Systems (CTMS) Market Trends Analysis (2020-2032)
7.3.2 Clinical Trial Management Systems (CTMS) Market Size Estimates and Forecasts to 2032 (USD Billion)
7.4 Clinical Analytics Platforms
7.4.1 Clinical Analytics Platforms Market Trends Analysis (2020-2032)
7.4.2 Clinical Analytics Platforms Market Size Estimates and Forecasts to 2032 (USD Billion)
7.5 Randomization and Trial Supply Management (RTSM)
7.5.1 Randomization and Trial Supply Management (RTSM) Market Trends Analysis (2020-2032)
7.5.2 Randomization and Trial Supply Management (RTSM) Market Size Estimates and Forecasts to 2032 (USD Billion)
7.6 Clinical Data Integration Platforms
7.6.1 Clinical Data Integration Platforms Market Trends Analysis (2020-2032)
7.6.2 Clinical Data Integration Platforms Market Size Estimates and Forecasts to 2032 (USD Billion)
7.7 Electronic Clinical Outcome Assessment (eCOA)
7.7.1 Electronic Clinical Outcome Assessment (eCOA) Market Trends Analysis (2020-2032)
7.7.2 Electronic Clinical Outcome Assessment (eCOA) Market Size Estimates and Forecasts to 2032 (USD Billion)
7.8 Safety Solutions
7.8.1 Safety Solutions Market Trends Analysis (2020-2032)
7.8.2 Safety Solutions Market Size Estimates and Forecasts to 2032 (USD Billion)
7.9 Electronic Trial Master File (eTMF)
7.9.1 Electronic Trial Master File (eTMF) Market Trends Analysis (2020-2032)
7.9.2 Electronic Trial Master File (eTMF) Market Size Estimates and Forecasts to 2032 (USD Billion)
7.10 Electronic Consent (eConsent)
7.10.1 Electronic Consent (eConsent) Market Trends Analysis (2020-2032)
7.10.2 Electronic Consent (eConsent) Market Size Estimates and Forecasts to 2032 (USD Billion)
8. eClinical Solutions Market Segmentation, By Delivery Mode
8.1 Chapter Overview
8.2 Web and Cloud based
8.2.1 Web and Cloud based Market Trends Analysis (2020-2032)
8.2.2 Web and Cloud based Market Size Estimates and Forecasts to 2032 (USD Billion)
8.3 On- Premise
8.3.1 On- Premise Market Trends Analysis (2020-2032)
8.3.2 On- Premise Market Size Estimates And Forecasts To 2032 (USD Billion)
9. eClinical Solutions Market Segmentation, by Development Phases
9.1 Chapter Overview
9.2 Phase I
9.2.1 Phase I Market Trends Analysis (2020-2032)
9.2.2 Phase I Market Size Estimates and Forecasts to 2032 (USD Billion)
9.3 Phase II
9.3.1 Phase II Market Trends Analysis (2020-2032)
9.3.2 Phase II Market Size Estimates and Forecasts to 2032 (USD Billion)
9.4 Phase III
9.4.1 Phase III Market Trends Analysis (2020-2032)
9.4.2 Phase III Market Size Estimates and Forecasts to 2032 (USD Billion)
9.5 Phase IV
9.5.1 Phase IV Market Trends Analysis (2020-2032)
9.5.2 Phase IV Market Size Estimates and Forecasts to 2032 (USD Billion)
10. eClinical Solutions Market Segmentation, By End-use
10.1 Chapter Overview
10.2 Hospitals/Healthcare providers
10.2.1 Hospitals/Healthcare providers Market Trends Analysis (2020-2032)
10.2.2 Hospitals/Healthcare providers Market Size Estimates and Forecasts to 2032 (USD Billion)
10.3 CROs
10.3.1 CROs Market Trends Analysis (2020-2032)
10.3.2 CROs Market Size Estimates and Forecasts to 2032 (USD Billion)
10.4 Academic Institutes
10.4.1 Academic Institutes Market Trends Analysis (2020-2032)
10.4.2 Academic Institutes Market Size Estimates and Forecasts to 2032 (USD Billion)
10.5 Pharma & Biotech Organizations
10.5.1 Pharma & Biotech Organizations Market Trends Analysis (2020-2032)
10.5.2 Pharma & Biotech Organizations Market Size Estimates and Forecasts to 2032 (USD Billion)
10.6 Medical Device Manufacturers
10.6.1 Medical Device Manufacturers Market Trends Analysis (2020-2032)
10.6.2 Medical Device Manufacturers Market Size Estimates and Forecasts to 2032 (USD Billion)
11. Regional Analysis
11.1 Chapter Overview
11.2 North America
11.2.1 Trends Analysis
11.2.2 North America eClinical Solutions Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
11.2.3 North America eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.2.4 North America eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.2.5 North America eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.2.6 North America eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.2.7 USA
11.2.7.1 USA eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.2.7.2 USA eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.2.7.3 USA eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.2.7.4 USA eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.2.8 Canada
11.2.8.1 Canada eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.2.8.2 Canada eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.2.8.3 Canada eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.2.8.4 Canada eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.2.9 Mexico
11.2.9.1 Mexico eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.2.9.2 Mexico eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.2.9.3 Mexico eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.2.9.4 Mexico eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion
11.3 Europe
11.3.1 Eastern Europe
11.3.1.1 Trends Analysis
11.3.1.2 Eastern Europe eClinical Solutions Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
11.3.1.3 Eastern Europe eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.3.1.4 Eastern Europe eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.3.1.5 Eastern Europe eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.3.1.6 Eastern Europe eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.3.1.7 Poland
11.3.1.7.1 Poland eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.3.1.7.2 Poland eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.3.1.7.3 Poland eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.3.1.7.4 Poland eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.3.1.8 Romania
11.3.1.8.1 Romania eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.3.1.8.2 Romania eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.3.1.8.3 Romania eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.3.1.8.4 Romania eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.3.1.9 Hungary
11.3.1.9.1 Hungary eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.3.1.9.2 Hungary eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.3.1.9.3 Hungary eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.3.1.9.4 Hungary eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.3.1.10 Turkey
11.3.1.10.1 Turkey eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.3.1.10.2 Turkey eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.3.1.10.3 Turkey eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.3.1.10.4 Turkey eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.3.1.11 Rest of Eastern Europe
11.3.1.11.1 Rest of Eastern Europe eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.3.1.11.2 Rest of Eastern Europe eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.3.1.11.3 Rest of Eastern Europe eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.3.1.11.4 Rest of Eastern Europe eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.3.2 Western Europe
11.3.2.1 Trends Analysis
11.3.2.2 Western Europe eClinical Solutions Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
11.3.2.3 Western Europe eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.3.2.4 Western Europe eClinical Solutions Market Estimates and Forecasts, By Development Phases (2020-2032) (USD Billion)
11.3.2.5 Western Europe eClinical Solutions Market Estimates and Forecasts, by Delivery Technology (2020-2032) (USD Billion)
11.3.2.6 Western Europe eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.3.2.7 Germany
11.3.2.7.1 Germany eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.3.2.7.2 Germany eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.3.2.7.3 Germany eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.3.2.7.4 Germany eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.3.2.8 France
11.3.2.8.1 France eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.3.2.8.2 France eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.3.2.8.3 France eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.3.2.8.4 France eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.3.2.9 UK
11.3.2.9.1 UK eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.3.2.9.2 UK eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.3.2.9.3 UK eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.3.2.9.4 UK eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.3.2.10 Italy
11.3.2.10.1 Italy eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.3.2.10.2 Italy eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.3.2.10.3 Italy eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.3.2.10.4 Italy eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.3.2.11 Spain
11.3.2.11.1 Spain eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.3.2.11.2 Spain eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.3.2.11.3 Spain eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.3.2.11.4 Spain eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.3.2.12 Netherlands
11.3.2.12.1 Netherlands eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.3.2.12.2 Netherlands eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.3.2.12.3 Netherlands eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.3.2.12.4 Netherlands eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.3.2.13 Switzerland
11.3.2.13.1 Switzerland eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.3.2.13.2 Switzerland eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.3.2.13.3 Switzerland eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.3.2.13.4 Switzerland eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.3.2.14 Austria
11.3.2.14.1 Austria eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.3.2.14.2 Austria eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.3.2.14.3 Austria eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.3.2.14.4 Austria eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.3.2.15 Rest of Western Europe
11.3.2.15.1 Rest of Western Europe eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.3.2.15.2 Rest of Western Europe eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.3.2.15.3 Rest of Western Europe eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.3.2.15.4 Rest of Western Europe eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.4 Asia Pacific
11.4.1 Trends Analysis
11.4.2 Asia Pacific eClinical Solutions Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
11.4.3 Asia Pacific eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.4.4 Asia Pacific eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.4.5 Asia Pacific eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.4.6 Asia Pacific eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.4.7 China
11.4.7.1 China eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.4.7.2 China eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.4.7.3 China eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.4.7.4 China eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.4.8 India
11.4.8.1 India eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.4.8.2 India eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.4.8.3 India eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.4.8.4 India eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.4.9 Japan
11.4.9.1 Japan eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.4.9.2 Japan eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.4.9.3 Japan eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.4.9.4 Japan eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.4.10 South Korea
11.4.10.1 South Korea eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.4.10.2 South Korea eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.4.10.3 South Korea eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.4.10.4 South Korea eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.4.11 Vietnam
11.4.11.1 Vietnam eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.4.11.2 Vietnam eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.4.11.3 Vietnam eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.4.11.4 Vietnam eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.4.12 Singapore
11.4.12.1 Singapore eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.4.12.2 Singapore eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.4.12.3 Singapore eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.4.12.4 Singapore eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.4.13 Australia
11.4.13.1 Australia eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.4.13.2 Australia eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.4.13.3 Australia eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.4.13.4 Australia eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.4.14 Rest of Asia Pacific
11.4.14.1 Rest of Asia Pacific eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.4.14.2 Rest of Asia Pacific eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.4.14.3 Rest of Asia Pacific eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.4.14.4 Rest of Asia Pacific eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.5 Middle East and Africa
11.5.1 Middle East
11.5.1.1 Trends Analysis
11.5.1.2 Middle East eClinical Solutions Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
11.5.1.3 Middle East eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.5.1.4 Middle East eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.5.1.5 Middle East eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.5.1.6 Middle East eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.5.1.7 UAE
11.5.1.7.1 UAE eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.5.1.7.2 UAE eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.5.1.7.3 UAE eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.5.1.7.4 UAE eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.5.1.8 Egypt
11.5.1.8.1 Egypt eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.5.1.8.2 Egypt eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.5.1.8.3 Egypt eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.5.1.8.4 Egypt eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.5.1.9 Saudi Arabia
11.5.1.9.1 Saudi Arabia eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.5.1.9.2 Saudi Arabia eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.5.1.9.3 Saudi Arabia eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.5.1.9.4 Saudi Arabia eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.5.1.10 Qatar
11.5.1.10.1 Qatar eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.5.1.10.2 Qatar eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.5.1.10.3 Qatar eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.5.1.10.4 Qatar eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.5.1.11 Rest of Middle East
11.5.1.11.1 Rest of Middle East eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.5.1.11.2 Rest of Middle East eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.5.1.11.3 Rest of Middle East eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.5.1.11.4 Rest of Middle East eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.5.2 Africa
11.5.2.1 Trends Analysis
11.5.2.2 Africa eClinical Solutions Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
11.5.2.3 Africa eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.5.2.4 Africa eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.5.2.5 Africa eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.5.2.6 Africa eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.5.2.7 South Africa
11.5.2.7.1 South Africa eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.5.2.7.2 South Africa eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.5.2.7.3 South Africa eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.5.2.7.4 South Africa eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.5.2.8 Nigeria
11.5.2.8.1 Nigeria eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.5.2.8.2 Nigeria eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.5.2.8.3 Nigeria eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.5.2.8.4 Nigeria eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.5.2.9 Rest of Africa
11.5.2.9.1 Rest of Africa eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.5.2.9.2 Rest of Africa eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.5.2.9.3 Rest of Africa eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.5.2.9.4 Rest of Africa eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.6 Latin America
11.6.1 Trends Analysis
11.6.2 Latin America eClinical Solutions Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
11.6.3 Latin America eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.6.4 Latin America eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.6.5 Latin America eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.6.6 Latin America eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.6.7 Brazil
11.6.7.1 Brazil eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.6.7.2 Brazil eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.6.7.3 Brazil eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.6.7.4 Brazil eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.6.8 Argentina
11.6.8.1 Argentina eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.6.8.2 Argentina eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.6.8.3 Argentina eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.6.8.4 Argentina eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.6.9 Colombia
11.6.9.1 Colombia eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.6.9.2 Colombia eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.6.9.3 Colombia eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.6.9.4 Colombia eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
11.6.10 Rest of Latin America
11.6.10.1 Rest of Latin America eClinical Solutions Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
11.6.10.2 Rest of Latin America eClinical Solutions Market Estimates and Forecasts, By Delivery Mode (2020-2032) (USD Billion)
11.6.10.3 Rest of Latin America eClinical Solutions Market Estimates and Forecasts, by Development Phases (2020-2032) (USD Billion)
11.6.10.4 Rest of Latin America eClinical Solutions Market Estimates and Forecasts, By End-use (2020-2032) (USD Billion)
12. Company Profiles
12.1 Fountayn (formerly Datatrak International, Inc.)
12.1.1 Company Overview
12.1.2 Financial
12.1.3 Product / Services Offered
12.1.4 SWOT Analysis
12.2 Oracle
12.2.1 Company Overview
12.2.2 Financial
12.2.3 Product / Services Offered
12.2.4 SWOT Analysis
12.3 Calyx (formerly part of Parexel International Corporation)
12.3.1 Company Overview
12.3.2 Financial
12.3.3 Product / Services Offered
12.3.4 SWOT Analysis
12.4 Medidata (Dassault Systèmes)
12.4.1 Company Overview
12.4.2 Financial
12.4.3 Product / Services Offered
12.4.4 SWOT Analysis
12.5 CRF Health (Signant Health)
12.5.1 Company Overview
12.5.2 Financial
12.5.3 Product / Services Offered
12.5.4 SWOT Analysis
12.6 Clario (ERT and Bioclinica)
12.6.1 Company Overview
12.6.2 Financial
12.6.3 Product / Services Offered
12.6.4 SWOT Analysis
12.7 eClinicalWorks
12.7.1 Company Overview
12.7.2 Financial
12.7.3 Product / Services Offered
12.7.4 SWOT Analysis
12.8 Merative (IBM Watson Health)
12.8.1 Company Overview
12.8.2 Financial
12.8.3 Product / Services Offered
12.8.4 SWOT Analysis
12.9 MaxisIT
12.9.1 Company Overview
12.9.2 Financial
12.9.3 Product / Services Offered
12.9.4 SWOT Analysis
12.10 IQVIA
12.10.1 Company Overview
12.10.2 Financial
12.10.3 Product / Services Offered
12.10.4 SWOT Analysis
13. Use Cases and Best Practices
14. Conclusion
An accurate research report requires proper strategizing as well as implementation. There are multiple factors involved in the completion of good and accurate research report and selecting the best methodology to compete the research is the toughest part. Since the research reports we provide play a crucial role in any company’s decision-making process, therefore we at SNS Insider always believe that we should choose the best method which gives us results closer to reality. This allows us to reach at a stage wherein we can provide our clients best and accurate investment to output ratio.
Each report that we prepare takes a timeframe of 350-400 business hours for production. Starting from the selection of titles through a couple of in-depth brain storming session to the final QC process before uploading our titles on our website we dedicate around 350 working hours. The titles are selected based on their current market cap and the foreseen CAGR and growth.
The 5 steps process:
Step 1: Secondary Research:
Secondary Research or Desk Research is as the name suggests is a research process wherein, we collect data through the readily available information. In this process we use various paid and unpaid databases which our team has access to and gather data through the same. This includes examining of listed companies’ annual reports, Journals, SEC filling etc. Apart from this our team has access to various associations across the globe across different industries. Lastly, we have exchange relationships with various university as well as individual libraries.

Step 2: Primary Research
When we talk about primary research, it is a type of study in which the researchers collect relevant data samples directly, rather than relying on previously collected data. This type of research is focused on gaining content specific facts that can be sued to solve specific problems. Since the collected data is fresh and first hand therefore it makes the study more accurate and genuine.
We at SNS Insider have divided Primary Research into 2 parts.
Part 1 wherein we interview the KOLs of major players as well as the upcoming ones across various geographic regions. This allows us to have their view over the market scenario and acts as an important tool to come closer to the accurate market numbers. As many as 45 paid and unpaid primary interviews are taken from both the demand and supply side of the industry to make sure we land at an accurate judgement and analysis of the market.
This step involves the triangulation of data wherein our team analyses the interview transcripts, online survey responses and observation of on filed participants. The below mentioned chart should give a better understanding of the part 1 of the primary interview.

Part 2: In this part of primary research the data collected via secondary research and the part 1 of the primary research is validated with the interviews from individual consultants and subject matter experts.
Consultants are those set of people who have at least 12 years of experience and expertise within the industry whereas Subject Matter Experts are those with at least 15 years of experience behind their back within the same space. The data with the help of two main processes i.e., FGDs (Focused Group Discussions) and IDs (Individual Discussions). This gives us a 3rd party nonbiased primary view of the market scenario making it a more dependable one while collation of the data pointers.
Step 3: Data Bank Validation
Once all the information is collected via primary and secondary sources, we run that information for data validation. At our intelligence centre our research heads track a lot of information related to the market which includes the quarterly reports, the daily stock prices, and other relevant information. Our data bank server gets updated every fortnight and that is how the information which we collected using our primary and secondary information is revalidated in real time.

Step 4: QA/QC Process
After all the data collection and validation our team does a final level of quality check and quality assurance to get rid of any unwanted or undesired mistakes. This might include but not limited to getting rid of the any typos, duplication of numbers or missing of any important information. The people involved in this process include technical content writers, research heads and graphics people. Once this process is completed the title gets uploader on our platform for our clients to read it.
Step 5: Final QC/QA Process:
This is the last process and comes when the client has ordered the study. In this process a final QA/QC is done before the study is emailed to the client. Since we believe in giving our clients a good experience of our research studies, therefore, to make sure that we do not lack at our end in any way humanly possible we do a final round of quality check and then dispatch the study to the client.
By Product
Electronic Data Capture (EDC) and Clinical Data Management Systems (CDMS)
Clinical Trial Management Systems (CTMS)
Clinical Analytics Platforms
Randomization and Trial Supply Management (RTSM)
Clinical Data Integration Platforms
Electronic Clinical Outcome Assessment (eCOA)
Safety Solutions
Electronic Trial Master File (eTMF)
Electronic Consent (eConsent)
By Delivery Mode
Web and Cloud based
On- Premise
By Development Phases
Phase I
Phase II
Phase III
Phase IV
By End-use
Hospitals/Healthcare providers
CROs
Academic Institutes
Pharma & Biotech Organizations
Medical Device Manufacturers
Request for Segment Customization as per your Business Requirement: Segment Customization Request
Regional Coverage:
North America
US
Canada
Mexico
Europe
Eastern Europe
Poland
Romania
Hungary
Turkey
Rest of Eastern Europe
Western Europe
Germany
France
UK
Italy
Spain
Netherlands
Switzerland
Austria
Rest of Western Europe
Asia Pacific
China
India
Japan
South Korea
Vietnam
Singapore
Australia
Rest of Asia Pacific
Middle East & Africa
Middle East
UAE
Egypt
Saudi Arabia
Qatar
Rest of Middle East
Africa
Nigeria
South Africa
Rest of Africa
Latin America
Brazil
Argentina
Colombia
Rest of Latin America
Request for Country Level Research Report: Country Level Customization Request
Available Customization
With the given market data, SNS Insider offers customization as per the company’s specific needs. The following customization options are available for the report:
Detailed Volume Analysis
Criss-Cross segment analysis (e.g. Product X Application)
Competitive Product Benchmarking
Geographic Analysis
Additional countries in any of the regions
Customized Data Representation
Detailed analysis and profiling of additional market players
The Single-Use Bioreactors Market Size was valued at USD 3872.40 million in 2023 and is expected to reach USD 13784.83 million by 2031 and grow at a CAGR of 17.2% over the forecast period 2024-2031.
The Physiotherapy Equipment Market Size was valued at USD 20.9 billion in 2023, is projected to grow at a CAGR of 6.9% to reach USD 38.2 billion by 2032.
The Medical Nonwoven Disposable Market was valued at USD 27.76 billion in 2023 and is expected to reach USD 75.29 billion by 2032, growing at a CAGR of 11.77% from 2024-2032.
The Mononucleosis Diagnostic Market size was estimated at USD 1.7 billion in 2022 and is expected to reach USD 3.2 billion by 2030 with a growing CAGR of 8.6% during the forecast period of 2023-2030.
The Alopecia Treatment Market Size was valued at USD 8.80 Billion in 2023, and is expected to reach USD 20.70 Billion by 2032, and grow at a CAGR of 10.48%.
The Doppler Ultrasound Market Size was valued at USD 1.83 Billion in 2023 and is expected to reach USD 2.53 Billion by 2032 and grow at a CAGR of 3.77% Over the Forecast Period of 2024-2032.
Hi! Click one of our member below to chat on Phone