Get More Information on Digital Clinical Trials Market - Request Sample Report
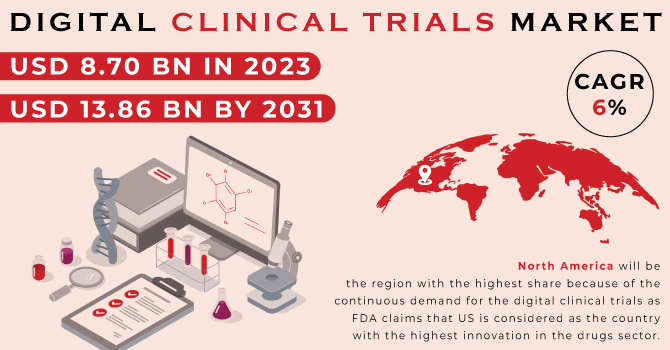
The Digital Clinical Trials Market was valued at USD 8.70 billion in 2023 and is expected to reach at USD 13.86 billion in 2031, and grow at a CAGR of 6 % over the forecast period of 2024-2031.
Digital term is familiar and is been utilized in almost every industry, with the rising demand for the digitalization because of the benefits associated with it the term digitalization will create a major difference in almost every industry, when we focus on healthcare industry there has been an immense growth in the technological advancement compared to the previous years, clinical trials are used in the healthcare industry is specifically used for the testing of the new drugs or any advancement on the humans to gain approval for that particular drug so that it can be used on a large population or targeted audience, Clinical trials in health care industry make it possible for the healthcare organization to focus on how the new invented drugs or surgical aspects works and what changes has to be made, The workshop held by NIH and NSF in 2019 regarding how digital can be implemented in the clinical trials was a positive factor for this market, FDA also plays a major part in approving the clinical trials regarding the drugs, there are certain guidelines and instructions by the FDA which every company or organization should follow in order to gain approval,
Digital Clinical trials has an opportunity to seek growth during the forecasted period digital includes the AI, data interpretation, health related data, digital retention. Digitalization in clinical trials can play a big role in terms of recording a patients data which includes heartrate, respiratory system, sleep which is an essential element when health related trails is considered, Also the fact that Covid 19 also created awareness regarding the digital clinical trials as the rules imposed by the government made it difficult to conduct human trials, Though the lack of awareness, and the adaptability amongst the stakeholders and the adaptability factor of switching from the basic standard to the digital clinical trials and the regulatory guidelines are the challenges the market might face during the forecasted period.
The lockdown rules imposed by the government made it difficult for the healthcare professionals to conduct the clinical trials, every industry was adapting the digitalization as there was no alternative, Covid-19 had a great impact on the digital clinical trial market, the need for the human trials was not possible because of the Covid guidelines and also the fear of the tangible access to the corona virus gave a boom for the digital clinical trials with the help of the digital clinical trials it becomes easy in terms of patients consent, through various forms of available platforms to communicate virtually, to record the data of patients, there are various applications and software which records the data of patients and which makes it easy for the patients to record and send the data accordingly without any physical contact, well there were many arguments regarding the limitations of the digital clinical trials and many other strategies which will be implemented for the scope and usage of the digital clinical trials. Thought the covid 19 impacted the digital clinical trials where it increased the awareness of the digitalization in clinical trials and also the benefits associated with it. The most beneficial factors which got introduced during the pandemic regarding the digital clinical trials included the teleconsultation which impacted the market as a fact that in virtual clinical trial it becomes easy for the patient. The report will give you brief information about what were the exact changes which tend to happen between the phase of pre-covid and post-covid.
Drivers
Initiatives taken by the key players all around the globe to invest in the digital clinical trials post covid as pandemic created awareness for the market.
The increasing demand for the digital clinical trials.
Restrains
The fact that it is costlier than the conventional clinical trials.
Opportunity
Initiative taken by the government and the healthcare organizations to create awareness about the digital clinical trials
The benefits associated with the digital clinical trials
Challenge
The adaptability issue and the perceptions of stakeholders and other healthcare professionals regarding the conventional trials.
North America will be the region with the highest share because of the continuous demand for the digital clinical trials as FDA claims that US is considered as the country with the highest innovation in the drugs sector.
APAC will be the region with the highest CAGR as the urbanization and the technological advancement in the emerging and the developed nations of this region are seeking continuous growth in healthcare sector.
Need any customization research on Digital Clinical Trials Market - Enquiry Now
The major key players are PPD, Inc, Stignant health, Human first, CRFweb, Data Management 365, IQVIA, IBM, Deloitte and Other Players.
PPD Inc -The ongoing R&D to study about the digital clinical trials and how it can be implemented.
Stignant -Launch of telemedicine platform innovations to optimize remote and decentralized clinical trial operations.
These reports will give you an exact brief about what are the recent developments of all the major key players listed in the report
| Report Attributes | Details |
| Market Size in 2023 | US$ 8.70 Bn |
| Market Size by 2030 | US$ 13.86 Bn |
| CAGR | CAGR of 6% From 2024 to 2031 |
| Base Year | 2023 |
| Forecast Period | 2024-2031 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Product type (Digital Wearable Devices, AI-Enabled Devices, Health App) • By Type (Decentralized/Virtual Clinical Trials, Hybrid Clinical Trials) • By Phase (Phase I, Phase II, Phase III, Phase IV) • By End User (Pharmaceutical companies, Biotechnology companies, Contract Research Organizations (CRO), Others) |
| Regional Analysis/Coverage | North America (USA, Canada, Mexico), Europe (Germany, UK, France, Italy, Spain, Netherlands, Rest of Europe), Asia-Pacific (Japan, South Korea, China, India, Australia, Rest of Asia-Pacific), The Middle East & Africa (Israel, UAE, South Africa, Rest of Middle East & Africa), Latin America (Brazil, Argentina, Rest of Latin America) |
| Company Profiles | PPD, Inc, Stignant health, Human first, CRFweb, Data Management 365, IQVIA, IBM, Deloitte, Others |
| Key Drivers | • Initiatives taken by the key players all around the globe to invest in the digital clinical trials post covid as pandemic created awareness for the market. • The increasing demand for the digital clinical trials. |
| Market Opportunities | • Initiative taken by the government and the healthcare organizations to create awareness about the digital clinical trials. • The benefits associated with the digital clinical trials |
Ans: The Digital Clinical Trials Market size was valued at US$ 8.7 Bn in 2023.
Initiatives taken by the key players all around the globe to invest in the digital clinical trials post covid as pandemic created awareness for the market.
The increasing demand for the digital clinical trials.Clinical Reference Laboratory, Corporation of America holdings, Quest Diagnostics Incorporated, Spectra Lab rotaries
Global digital clinical trial market is divided in four segments.
Ans: The Digital Clinical Trials Market is to grow at a CAGR of 6% Over the Forecast Period 2024-2031.
Table of Contents
1.Introduction
1.1 Market Definition
1.2 Scope
1.3 Research Assumptions
2.Research Methodology
3.Market Dynamics
3.1 Drivers
3.2 Restraints
3.3 Opportunities
3.4 Challenges
4.Impact Analysis
4.1 COVID 19 Impact Analysis
5.Value Chain Analysis
6.Porter’s 5 forces model
7.PEST Analysis
8.By Product type
8.1 Digital Wearable Devices
8.2 AI-Enabled Devices
8.3 Health Apps
9.By Type
9.1 Decentralized/Virtual Clinical Trials
9.2 Hybrid Clinical Trials
10.By Phase
10.1 Phase I
10.2 Phase II
10.3 Phase III
10.4 Phase IV
11.By End User
11.1 Pharmaceutical companies
11.2 Biotechnology companies
11.3 Contract Research Organizations (CRO)
11.4 Others
12.Regional Analysis
12.1 Introduction
12.2 North America
12.2.1 North America Global Mental Health Product Market by country
12.2.2North America Global Mental Health Product Market by Product Type
12.2.3 North America Global Mental Health Product Market by Type
12.2.4 North America Global Mental Health Product Market by Phase
12.2.5 North America Global Mental Health Product Market by End User
12.2.6 USA
12.2.6.1 USA Global Mental Health Product Market by Product Type
12.2.6.2 USA Global Mental Health Product Market by Type
12.2.6.3 USA Global Mental Health Product Market by Phase
12.2.6.4 USA Global Mental Health Product Market by End User
12.2.7 Canada
12.2.7.1 Canada Global Mental Health Product Market by Product Type
12.2.7.2 Canada Global Mental Health Product Market by Type
12.2.7.3 Canada Global Mental Health Product Market by Phase
12.2.7.4 Canada Global Mental Health Product Market by End User
12.2.8 Mexico
12.2.8.1 Mexico Global Mental Health Product Market by Product Type
12.2.8.2 Mexico Global Mental Health Product Market by Type
12.2.8.3 Mexico Global Mental Health Product Market by Phase
12.2.8.4 Mexico Global Mental Health Product Market by End User
12.3 Europe
12.3.1 Europe Global Mental Health Product Market by country
1.3.3.2 Europe Global Mental Health Product Market by Product Type
12.3.3 Europe Global Mental Health Product Market by Type
12.3.4 Europe Global Mental Health Product Market by Phase
12.3.5 Europe Global Mental Health Product Market by End User
12.3.6 Germany
12.3.6.1 Germany Global Mental Health Product Market by Product Type
12.3.6.2 Germany Global Mental Health Product Market by Type
12.3.6.3 Germany Global Mental Health Product Market by Phase
12.3.6.4 Germany Global Mental Health Product Market by End User
12.3.7 UK
12.3.7.1 UK Global Mental Health Product Market by Product Type
12.3.7.2 UK Global Mental Health Product Market by Type
12.3.7.3 UK Global Mental Health Product Market by Phase
12.3.7.4 UK Global Mental Health Product Market by End User
12.3.8 France
12.3.8.1 France Global Mental Health Product Market by Product Type
12.3.8.2 France Global Mental Health Product Market by Type
12.3.8.3 France Global Mental Health Product Market by Phase
12.3.8.4 France Global Mental Health Product Market by End User
12.3.9 Italy
12.3.9.1 Italy Global Mental Health Product Market by Product Type
12.3.9.2 Italy Global Mental Health Product Market by Type
12.3.9.3 Italy Global Mental Health Product Market by Phase
12.3.9.4 Italy Global Mental Health Product Market by End User
12.3.10 Spain
12.3.10.1 Spain Global Mental Health Product Market by Product Type
12.3.10.2 Spain Global Mental Health Product Market by Type
12.3.10.3 Spain Global Mental Health Product Market by Phase
12.3.10.4 Spain Global Mental Health Product Market by End User
12.3.11 The Netherlands
12.3.11.1 Netherlands Global Mental Health Product Market by Product Type
12.3.11.2 Netherlands Global Mental Health Product Market by Type
12.3.11.3 Netherlands Global Mental Health Product Market by Phase
12.3.11.4 Netherlands Global Mental Health Product Market by End User
12.3.12 Rest of Europe
12.3.12.1 Rest of Europe Global Mental Health Product Market by Product Type
12.3.12.2 Rest of Europe Global Mental Health Product Market by Type
12.3.12.3 Rest of Europe Global Mental Health Product Market by Phase
12.3.12.4 Rest of Europe Global Mental Health Product Market by End User
12.4 Asia-Pacific
12.4.1 Asia Pacific Global Mental Health Product Market by country
12.4.2 Asia Pacific Global Mental Health Product Market by Product Type
12.4.3 Asia Pacific Global Mental Health Product Market by Type
12.4.4Asia Pacific Global Mental Health Product Market by Phase
12.4.5Asia Pacific Global Mental Health Product Market by End User
12.4.6 Japan
12.4.6.1 Japan Global Mental Health Product Market by Product Type
12.4.6.2 Japan Global Mental Health Product Market by Type
12.4.6.3 Japan Global Mental Health Product Market by Phase
12.4.6.4 Japan Global Mental Health Product Market by End User
12.4.7 South Korea
12.4.7.1 South Korea Global Mental Health Product Market by Product Type
12.4.7.2 South Korea Global Mental Health Product Market by Type
12.4.7.3 South Korea Global Mental Health Product Market by Phase
12.4.7.4 South Korea Global Mental Health Product Market by End User
12.4.8 China
12.4.8.1 China Global Mental Health Product Market by Product Type
12.4.8.2 China Global Mental Health Product Market by Type
12.4.8.3 China Global Mental Health Product Market by Phase
12.4.8.4 China Global Mental Health Product Market by End User
12.4.9 India
12.4.9.1 India Global Mental Health Product Market by Product Type
12.4.9.2 India Global Mental Health Product Market by Type
12.4.9.3 India Global Mental Health Product Market by Phase
12.4.9.4 India Global Mental Health Product Market by End User
12.4.10 Australia
12.4.10.1 Australia Global Mental Health Product Market by Product Type
12.4.10.2 Australia Global Mental Health Product Market by Type
12.4.10.3 Australia Global Mental Health Product Market by Phase
12.4.10.4 Australia Global Mental Health Product Market by End User
12.4.11 Rest of Asia-Pacific
12.4.11.1 APAC Global Mental Health Product Market by Product Type
12.4.11.2 APAC Global Mental Health Product Market by Type
12.4.11.3 APAC Global Mental Health Product Market by Phase
12.4.11.4 APAC Global Mental Health Product Market by End User
12.5 The Middle East & Africa
12.5.1 The Middle East & Africa Global Mental Health Product Market by country
12.5.2 The Middle East & Africa Global Mental Health Product Market by Product Type
12.5.3 The Middle East & Africa Global Mental Health Product Market by Type
12.5.4The Middle East & Africa Global Mental Health Product Market by Phase
12.5.5 The Middle East & Africa Global Mental Health Product Market by End User
12.5.6 Israel
12.5.6.1 Israel Global Mental Health Product Market by Product Type
12.5.6.2 Israel Global Mental Health Product Market by Type
12.5.6.3 Israel Global Mental Health Product Market by Phase
12.5.6.4 Israel Global Mental Health Product Market by End User
12.5.7 UAE
12.5.7.1 UAE Global Mental Health Product Market by Product Type
12.5.7.2 UAE Global Mental Health Product Market by Type
12.5.7.3 UAE Global Mental Health Product Market by Phase
12.5.7.4 UAE Global Mental Health Product Market by End User
12.5.8 South Africa
12.5.8.1 South Africa Global Mental Health Product Market by Product Type
12.5.8.2 South Africa Global Mental Health Product Market by Type
12.5.8.3 South Africa Global Mental Health Product Market by Phase
12.5.8.4 South Africa Global Mental Health Product Market by End User
12.5.9 Rest of Middle East & Africa
12.5.9.1 Rest of Middle East & Asia Global Mental Health Product Market by Product Type
12.5.9.2 Rest of Middle East & Asia Global Mental Health Product Market by Type
12.5.9.3 Rest of Middle East & Asia Global Mental Health Product Market Phase
12.5.9.4 Rest of Middle East & Asia Global Mental Health Product Market by End User
12.6 Latin America
12.6.1 Latin America Global Mental Health Product Market by country
12.6.2 Latin America Global Mental Health Product Market by Product Type
12.6.3 Latin America Global Mental Health Product Market by Type
12.6.4 Latin America Global Mental Health Product Market by Phase
12.6.5 Latin America Global Mental Health Product Market by End User
12.6.6 Brazil
12.6.6.1 Brazil Global Mental Health Product Market by Product Type
12.6.6.2 Brazil Africa Global Mental Health Product Market by Type
12.6.6.3 Brazil Global Mental Health Product Market by Phase
12.6.6.4 Brazil Global Mental Health Product Market by End User
12.6.7 Argentina
12.6.7.1 Argentina Global Mental Health Product Market by Product Type
12.6.7.2 Argentina Global Mental Health Product Market by Type
12.6.7.3 Argentina Global Mental Health Product Market by Phase
12.6.7.4 Argentina Global Mental Health Product Market by End User
12.6.8 Rest of Latin America
12.6.8.1 Rest of Latin America Global Mental Health Product Market by Product Type
12.6.8.2 Rest of Latin America Global Mental Health Product Market by Type
12.6.8.3 Rest of Latin America Global Mental Health Product Market by Phase
12.6.8.4 Rest of Latin America Global Mental Health Product Market by End User
13.Company Profile
13.1 PPD, Inc
13.1.1 Market Overview
13.1.2 Financials
13.1.3 Product/Services/Offerings
13.1.4 SWOT Analysis
13.1.5 The SNS View
13.2 Stignant health
13.2.1 Market Overview
13.2.2 Financials
13.2.3 Product/Services/Offerings
13.2.4 SWOT Analysis
13.2.5 The SNS View
13.3 Human first
13.3.1 Market Overview
13.3.2 Financials
13.3.3 Product/Services/Offerings
13.3.4 SWOT Analysis
13.3.5 The SNS View
13.4 CRFweb
13.4.1 Market Overview
13.4.2 Financials
13.4.3 Product/Services/Offerings
13.4.4 SWOT Analysis
13.4.5 The SNS View
13.5 Data Management 365
13.5.1 Market Overview
13.5.2 Financials
13.5.3 Product/Services/Offerings
13.5.4 SWOT Analysis
13.5.5 The SNS View
13.6 IQVIA
13.6.1 Market Overview
13.6.2 Financials
13.6.3 Product/Services/Offerings
13.6.4 SWOT Analysis
13.6.5 The SNS View
13.6 IBM
13.6.1 Market Overview
13.6.2 Financials
13.6.3 Product/Services/Offerings
13.6.4 SWOT Analysis
13.6.5 The SNS View
13.6 Deloitte
13.6.1 Market Overview
13.6.2 Financials
13.6.3 Product/Services/Offerings
13.6.4 SWOT Analysis
13.6.5 The SNS View
13.6 Others
13.6.1 Market Overview
13.6.2 Financials
13.6.3 Product/Services/Offerings
13.6.4 SWOT Analysis
13.6.5 The SNS View
14. Competitive Landscape
14.1 Competitive Benchmarking
14.2 Market Share Analysis
14.3 Recent Developments
15. Used cases and Best Practices
16. Conclusion
An accurate research report requires proper strategizing as well as implementation. There are multiple factors involved in the completion of good and accurate research report and selecting the best methodology to compete the research is the toughest part. Since the research reports we provide play a crucial role in any company’s decision-making process, therefore we at SNS Insider always believe that we should choose the best method which gives us results closer to reality. This allows us to reach at a stage wherein we can provide our clients best and accurate investment to output ratio.
Each report that we prepare takes a timeframe of 350-400 business hours for production. Starting from the selection of titles through a couple of in-depth brain storming session to the final QC process before uploading our titles on our website we dedicate around 350 working hours. The titles are selected based on their current market cap and the foreseen CAGR and growth.
The 5 steps process:
Step 1: Secondary Research:
Secondary Research or Desk Research is as the name suggests is a research process wherein, we collect data through the readily available information. In this process we use various paid and unpaid databases which our team has access to and gather data through the same. This includes examining of listed companies’ annual reports, Journals, SEC filling etc. Apart from this our team has access to various associations across the globe across different industries. Lastly, we have exchange relationships with various university as well as individual libraries.

Step 2: Primary Research
When we talk about primary research, it is a type of study in which the researchers collect relevant data samples directly, rather than relying on previously collected data. This type of research is focused on gaining content specific facts that can be sued to solve specific problems. Since the collected data is fresh and first hand therefore it makes the study more accurate and genuine.
We at SNS Insider have divided Primary Research into 2 parts.
Part 1 wherein we interview the KOLs of major players as well as the upcoming ones across various geographic regions. This allows us to have their view over the market scenario and acts as an important tool to come closer to the accurate market numbers. As many as 45 paid and unpaid primary interviews are taken from both the demand and supply side of the industry to make sure we land at an accurate judgement and analysis of the market.
This step involves the triangulation of data wherein our team analyses the interview transcripts, online survey responses and observation of on filed participants. The below mentioned chart should give a better understanding of the part 1 of the primary interview.

Part 2: In this part of primary research the data collected via secondary research and the part 1 of the primary research is validated with the interviews from individual consultants and subject matter experts.
Consultants are those set of people who have at least 12 years of experience and expertise within the industry whereas Subject Matter Experts are those with at least 15 years of experience behind their back within the same space. The data with the help of two main processes i.e., FGDs (Focused Group Discussions) and IDs (Individual Discussions). This gives us a 3rd party nonbiased primary view of the market scenario making it a more dependable one while collation of the data pointers.
Step 3: Data Bank Validation
Once all the information is collected via primary and secondary sources, we run that information for data validation. At our intelligence centre our research heads track a lot of information related to the market which includes the quarterly reports, the daily stock prices, and other relevant information. Our data bank server gets updated every fortnight and that is how the information which we collected using our primary and secondary information is revalidated in real time.

Step 4: QA/QC Process
After all the data collection and validation our team does a final level of quality check and quality assurance to get rid of any unwanted or undesired mistakes. This might include but not limited to getting rid of the any typos, duplication of numbers or missing of any important information. The people involved in this process include technical content writers, research heads and graphics people. Once this process is completed the title gets uploader on our platform for our clients to read it.
Step 5: Final QC/QA Process:
This is the last process and comes when the client has ordered the study. In this process a final QA/QC is done before the study is emailed to the client. Since we believe in giving our clients a good experience of our research studies, therefore, to make sure that we do not lack at our end in any way humanly possible we do a final round of quality check and then dispatch the study to the client.
By Product type
Digital Wearable Devices
AI-Enabled Devices
Health Apps
By Type
Decentralized/Virtual Clinical Trials
Hybrid Clinical Trials
By Phase
Phase I
Phase II
Phase III
Phase IV
By End User
Pharmaceutical companies
Biotechnology companies
Contract Research Organizations (CRO)
Others
Request for Segment Customization as per your Business Requirement: Segment Customization Request
REGIONAL COVERAGE:
North America
US
Canada
Mexico
Europe
Eastern Europe
Poland
Romania
Hungary
Turkey
Rest of Eastern Europe
Western Europe
Germany
France
UK
Italy
Spain
Netherlands
Switzerland
Austria
Rest of Western Europe
Asia Pacific
China
India
Japan
South Korea
Vietnam
Singapore
Australia
Rest of Asia Pacific
Middle East & Africa
Middle East
UAE
Egypt
Saudi Arabia
Qatar
Rest of the Middle East
Africa
Nigeria
South Africa
Rest of Africa
Latin America
Brazil
Argentina
Colombia
Request for Country Level Research Report: Country Level Customization Request
Available Customization
With the given market data, SNS Insider offers customization as per the company’s specific needs. The following customization options are available for the report:
Product Analysis
Criss-Cross segment analysis (e.g. Product X Application)
Product Matrix which gives a detailed comparison of product portfolio of each company
Geographic Analysis
Additional countries in any of the regions
Company Information
Detailed analysis and profiling of additional market players (Up to five)
The Stem Cell Therapy Market Size was valued at USD 287 million in 2023 and is witnessed to reach USD 1,113.12 million by 2032 and grow at a CAGR of 17.10% over the forecast period 2024-2032.
The Single-Use Bioreactors Market Size was valued at USD 3872.40 million in 2023 and is expected to reach USD 13784.83 million by 2031 and grow at a CAGR of 17.2% over the forecast period 2024-2031.
The Smart Contact Lenses Market size was USD 6.33 billion in 2023, projected to hit USD 14.94 billion by 2032, growing at 10.03% CAGR.
The Cell Signaling Market Size was valued at USD 5.1 billion in 2023 and is expected to reach USD 9.4 billion by 2032 and grow at a CAGR of 7.1% over the forecast period 2024-2032.
The Electric Wheelchair Market was valued at USD 3.66 billion in 2023 and is expected to reach USD 9.82 billion by 2032, growing at a CAGR of 11.50% from 2024 to 2032.
The Diet Pills Market size is expected to reach USD 3.77 Bn by 2032 and was valued at USD 1.61 Bn in 2023, the CAGR is expected to be 9.9% over the forecast period of 2024-2032.
Hi! Click one of our member below to chat on Phone