Get More Information on Colorectal Cancer Therapeutics Market - Request Sample Report
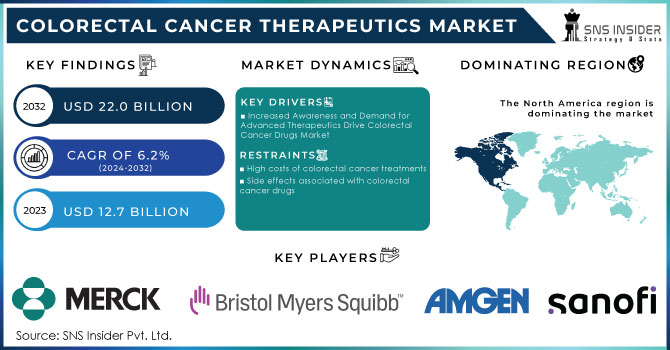
The Colorectal Cancer Therapeutics Market was valued at USD 12.7 billion in 2023 and is expected to reach USD 22.0 billion by 2032 and grow at a CAGR of 6.2% over the forecast period 2024-2032.
Colorectal cancer (CRC) therapeutics market is growing globally due to such various reasons as increased prevalence, advancements in diagnostics and treatments, and increased awareness. According to a study published in the National Library of Medicine in 2021, CRC accounted for an approximate 10% distribution of global cancer cases and 9.4% of the global number of cancer deaths in 2020; the cancer ranked as the second deadliest after lung cancer. The WHO recently reported almost 2 million CRC cases worldwide in 2020. In the U.S., the American Cancer Society projects that 1 in 25 women and 1 in 23 men will develop CRC during their lifetime.
Advances in screening and diagnostics have contributed to a declining CRC mortality while better treatments also contribute to an important factor. In June 2023, the US FDA approved FoundationOne Liquid CDx, which is a genomic profiling test that is designed to identify patients with metastatic CRC harboring BRAF V600E alterations who may benefit from BRAFTOVI and cetuximab therapy. The growing acceptability of advanced therapies such as Avastin, Erbitux, and Stivarga also supports the growth of this market.
Competition and demand are likely to increase, especially due to the patent expirations of biologics, including Avastin, which expired in the United States in 2019 and in Europe in 2022. Biosimilars have entered the oncology space with the introduction of cost-effective alternatives such as Amgen and Allergan's Mvasi. As reported by AJMC, 20 oncology biologics are expected to lose exclusivity by 2023, hence propelling growth in the CRC therapeutics market.
Furthermore, the awareness of CRC coupled with government initiatives for early diagnosis is creating a conducive environment for market expansion. An upward trend will continue to prevail among the advanced treatments and therapies related to the disease in regions like Germany, the U.K., and China, thereby offering opportunities for market players.
Table 1: Recent Clinical Trials in Colorectal Cancer
| Trial Name | Drug Name | Company Name | Phase | Trial Status |
|
APEC |
Atezolizumab |
Roche |
Phase 3 |
Active |
|
DETERMINATION |
Nivolumab |
Bristol-Myers Squibb |
Phase 3 |
Recruiting |
|
CHRONOS |
Regorafenib |
Bayer |
Phase 3 |
Completed |
|
TRIO-012 |
Panitumumab |
Amgen |
Phase 2 |
Active |
|
COLO-REG |
TAS-102 |
Taiho Pharmaceutical |
Phase 3 |
Active |
Table 2: Targeted Therapies for Colorectal Cancer
| Drug Name | Target | Company Name | Trial Name | Phase |
|
Cetuximab |
EGFR |
Merck & Co. |
CRYSTAL |
Phase 3 |
|
Regorafenib |
Multiple kinases |
Bayer |
RESILIENCE |
Phase 3 |
|
Fractalkine |
CX3CR1 |
Axcella Health |
AXS-05 |
Phase 2 |
|
Sunitinib |
VEGFR, PDGFR |
Pfizer |
RESIST |
Phase 2 |
|
Trastuzumab |
HER2 |
Genentech |
HERACLES |
Phase 3 |
Drivers
Increased Awareness and Demand for Advanced Therapeutics Drive Colorectal Cancer Drugs Market
Increasing awareness of the disease and growing demand for high-tech effective treatment options are driving the global colorectal cancer drugs market. Colorectal cancer is an expensive cancer in terms of mortality. Increasing demand for novel therapeutics that may provide better treatment outcomes among patients diagnosed with colorectal cancer has increased further. A multi-agent approach to treatment is mostly required, involving a combination of drugs, as innovations in drug development continue. The various crucial market players launched several new drugs to cater to the different needs of patients suffering from colorectal cancer. For instance, with the introduction of the Young-Onset Colorectal Cancer Center by Dana-Farber in April 2019 as an improvement measure for patient care, the market is further expanded.
Despite the development of different drugs or treatments, the incidence of colorectal cancer has been increasing steadily and thus constitutes an unmet need in the market because of the requirement for advanced therapies. Although inexpensive generic chemotherapy drugs are available, the disease is complex and severe, so continuous innovation in therapeutics is required. This demand in favor of more effective drugs is a key driver in the global market for colorectal cancer medications.
Restraints
High costs of colorectal cancer treatments
Side effects associated with colorectal cancer drugs
By Drug Class
Based on drug class, the colorectal cancer drugs market is diversified into chemotherapy, immunotherapy, and others. Immunotherapy accounted for 83.0% of total revenue in 2023 and is expected to grow at the highest CAGR of 5.3% during the forecast period. Immunotherapies are increasingly preferred because they have fewer adverse effects compared with chemotherapy. Such factors as chemotherapy ineffectiveness, side effects of toxicity, and even resistance development are driving immunotherapy to new heights.
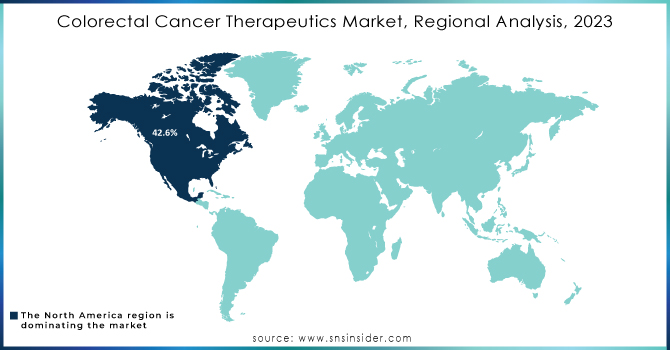
In 2023, North America accounted for the highest revenue share of 42.6% in colorectal cancer drugs. The region's market growth is to remain stable, due to a high incidence of CRC, an increase in treatment rates, and elevated drug prices compared with other regions. Lifestyle factors, such as increasing alcohol consumption, also add to the trend. The International Agency for Research on Cancer (IARC) estimated that about 60% of adults consumed alcohol in 2021, a risk factor that is linked to higher CRC incidence.
Asia Pacific is set to expand with the highest CAGR of 6.7% over the forecast period. This growth is attributed to the rising geriatric population and the growing involvement of public and private organizations in awareness programs about colorectal cancer. For example, a study published by the National Library of Medicine in April 2022 reported various pilot population-based CRC screening programs implemented in different administrative divisions of China, which would help in the early detection and management of the disease.
Need Any Customization Research On Colorectal Cancer Therapeutic Market - Inquiry Now
Key Drug Manufacturers for The Colorectal Cancer Therapeutic Market
F. Hoffmann-La Roche Ltd.
Amgen, Inc.
Bayer AG
Sanofi
Genentech, Inc.
Pfizer Inc.
Teva Pharmaceutical Industries Ltd.
Taiho Pharmaceutical (Otsuka Pharmaceutical Co., Ltd.)
Regeneron Pharmaceuticals, Inc.
Ipsen Biopharmaceuticals, Inc.
Key Drug Adjuvant Manufacturers for The Colorectal Cancer Therapeutic Market
Bristol-Myers Squibb
Merck & Co.
Amgen
Genentech (Roche)
Bayer
Taiho Pharmaceutical
Eli Lilly
Pfizer
Servier Pharmaceuticals
Johnson & Johnson
Key API Manufacturers for The Colorectal Cancer Therapeutic Market
Teva Pharmaceutical Industries Ltd.
Novartis AG
Bristol-Myers Squibb
F. Hoffmann-La Roche AG
Mylan N.V. (now part of Viatris)
Aurobindo Pharma
Zydus Cadila
Sun Pharmaceutical Industries Ltd.
Hikma Pharmaceuticals PLC
Apotex Inc.
Glenmark Pharmaceuticals
Sandoz (a Novartis division)
Eisai Co., Ltd.
Hetero Labs Limited
Cambrex Corporation
April 2024: Taiho Pharmaceutical Co., Ltd. announced promising results from a Phase III clinical trial of its drug LONSURF (trifluridine/tipiracil) for patients with metastatic colorectal cancer, showing improved overall survival rates compared to standard therapies.
March 2024: Roche reported advancements in its clinical studies evaluating the combination of Cotellic (cobimetinib) with immuno-oncologic agents, revealing encouraging outcomes in patients with advanced colorectal cancer.
February 2024: Regeneron Pharmaceuticals, Inc. initiated a new trial focusing on the efficacy of its PD-1 inhibitor, Libtayo (cemiplimab), in combination with chemotherapy for the treatment of patients with microsatellite instability-high (MSI-H) colorectal cancer.
January 2024: Akeda entered into a partnership with Hutchmed, acquiring commercial rights for the colorectal cancer drug fruquintinib outside China, aiming to expand its availability in international markets.
December 2023: Bristol-Myers Squibb received FDA approval for a new indication of Opdivo (nivolumab) as a treatment for patients with previously treated metastatic colorectal cancer with specific biomarkers.
November 2023: Amgen announced positive Phase II trial results for its investigational drug, which targets specific genetic mutations associated with colorectal cancer, paving the way for future studies and potential market entry.
In January 2023, Akeda, a company based in Japan, partnered with Hutchmed, located in Hong Kong, to acquire commercial rights for the colorectal cancer drug fruquintinib outside of China. Additionally, Roche is assessing the combination of its targeted therapy, Cotellic, with immuno-oncologic agents for CRC treatment. These developments are expected to stimulate innovation and drive growth in the market.
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | US$ 12.7 Billion |
| Market Size by 2032 | US$ 22.0 billion |
| CAGR | CAGR of 6.2% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Drug Class (Immunotherapy, Chemotherapy, Others) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | Merck & Co., Inc., F. Hoffmann-La Roche Ltd., Bristol-Myers Squibb Company, Amgen, Inc., Bayer AG, Sanofi, Genentech, Inc., Eli Lilly and Company, Pfizer Inc., Teva Pharmaceutical Industries Ltd., Taiho Pharmaceutical (Otsuka Pharmaceutical Co., Ltd.), Regeneron Pharmaceuticals, Inc., Novartis AG, and others. |
| Key Drivers | • Increased Awareness and Demand for Advanced Therapeutics Drive Colorectal Cancer Drugs Market |
| Restraints | • High costs of colorectal cancer treatments • Side effects associated with colorectal cancer drugs |
Ans: The estimated compound annual growth rate is 6.2% during the forecast period for the Colorectal Cancer Therapeutics market.
Ans: The projected market value of the Colorectal Cancer Therapeutics market is estimated at USD 12.7 billion in 2023 and is expected to reach USD 22.0 billion by 2032.
Ans: The increased awareness and demand for advanced therapeutics drive the colorectal cancer drugs market.
Ans: The high costs of colorectal cancer treatments are the restraints on the Colorectal Cancer Therapeutics market
Ans: North America is the dominant region with a 42.6% share in the Colorectal Cancer Therapeutics market.
Table of Contents
1. Introduction
1.1 Market Definition
1.2 Scope (Inclusion and Exclusions)
1.3 Research Assumptions
2. Executive Summary
2.1 Market Overview
2.2 Regional Synopsis
2.3 Competitive Summary
3. Research Methodology
3.1 Top-Down Approach
3.2 Bottom-up Approach
3.3. Data Validation
3.4 Primary Interviews
4. Market Dynamics Impact Analysis
4.1 Market Driving Factors Analysis
4.1.1 Drivers
4.1.2 Restraints
4.1.3 Opportunities
4.1.4 Challenges
4.2 PESTLE Analysis
4.3 Porter’s Five Forces Model
5. Statistical Insights and Trends Reporting
5.1 Incidence and Prevalence (2023)
5.2 Prescription Trends, (2023), by Region
5.3 Drug Volume: Production and usage volumes of pharmaceuticals.
5.4 Healthcare Spending: Expenditure data by government, insurers, and out-of-pocket by patients.
6. Competitive Landscape
6.1 List of Major Companies, By Region
6.2 Market Share Analysis, By Region
6.3 Product Benchmarking
6.3.1 Product specifications and features
6.3.2 Pricing
6.4 Strategic Initiatives
6.4.1 Marketing and promotional activities
6.4.2 Distribution and Supply Chain Strategies
6.4.3 Expansion plans and new product launches
6.4.4 Strategic partnerships and collaborations
6.5 Technological Advancements
6.6 Market Positioning and Branding
7. Colorectal Cancer Therapeutics Market Segmentation, by Drug Class
7.2 Immunotherapy
7.2.1 Immunotherapy Market Trends Analysis (2020-2032)
7.2.2 Immunotherapy Market Size Estimates and Forecasts to 2032 (USD Million)
7.3 Chemotherapy
7.3.1 Chemotherapy Market Trends Analysis (2020-2032)
7.3.2 Chemotherapy Market Size Estimates and Forecasts to 2032 (USD Million)
7.4 Others
7.4.1 Others Market Trends Analysis (2020-2032)
7.4.2 Others Market Size Estimates and Forecasts to 2032 (USD Million)
8. Regional Analysis
8.1 Chapter Overview
8.2 North America
8.2.1 Trends Analysis
8.2.2 North America Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Country (2020-2032) (USD Million)
8.2.3 North America Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.2.2 USA
8.2.2.1 USA Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.2.3 Canada
8.2.3.1 Canada Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.2.4 Mexico
8.2.4.1 Mexico Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.3 Europe
8.3.1 Eastern Europe
8.3.1.1 Trends Analysis
8.3.1.2 Eastern Europe Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Country (2020-2032) (USD Million)
8.3.1.3 Eastern Europe Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.3.1.4 Poland
8.3.1.4.1 Poland Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.3.1.5 Romania
8.3.1.5.1 Romania Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.3.1.6 Hungary
10.3.1.8.1 Hungary Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.3.1.7 Turkey
8.3.1.7.1 Turkey Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.3.1.8 Rest of Eastern Europe
8.3.1.8.1 Rest of Eastern Europe Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.3.2 Western Europe
8.3.2.1 Trends Analysis
8.3.2.2 Western Europe Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Country (2020-2032) (USD Million)
8.3.2.3 Western Europe Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.3.2.4 Germany
8.3.2.4.1 Germany Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.3.2.5 France
8.3.2.5.1 France Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.3.2.6 UK
8.3.2.6.1 UK Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.3.2.7 Italy
8.3.2.7.1 Italy Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.3.2.8 Spain
8.3.2.8.1 Spain Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.3.2.9 Netherlands
8.3.2.9.1 Netherlands Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.3.2.10 Switzerland
8.3.2.10.1 Switzerland Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.3.2.11 Austria
8.3.2.11.1 Austria Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.3.2.12 Rest of Western Europe
8.3.2.12.1 Rest of Western Europe Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.4 Asia Pacific
8.4.1 Trends Analysis
8.4.2 Asia Pacific Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Country (2020-2032) (USD Million)
8.4.3 Asia Pacific Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.4.4 China
8.4.4.1 China Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.4.5 India
8.4.5.1 India Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.4.6 Japan
8.4.6.1 Japan Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.4.7 South Korea
8.4.7.1 South Korea Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.4.8 Vietnam
8.4.8.1 Vietnam Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.4.9 Singapore
8.4.9.1 Singapore Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.4.10 Australia
8.4.10.1 Australia Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.4.11 Rest of Asia Pacific
8.4.11.1 Rest of Asia Pacific Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.5 Middle East and Africa
8.5.1 Middle East
8.5.1.1 Trends Analysis
8.5.1.2 Middle East Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Country (2020-2032) (USD Million)
10.5.1.3 Middle East Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.5.1.4 UAE
8.5.1.4.1 UAE Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.5.1.5 Egypt
8.5.1.5.1 Egypt Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.5.1.6 Saudi Arabia
8.5.1.6.1 Saudi Arabia Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.5.1.7 Qatar
8.5.1.7.1 Qatar Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.5.1.8 Rest of Middle East
8.5.1.8.1 Rest of Middle East Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.5.2 Africa
8.5.2.1 Trends Analysis
8.5.2.2 Africa Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Country (2020-2032) (USD Million)
8.5.2.3 Africa Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.5.2.4 South Africa
8.5.2.4.1 South Africa Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.5.2.5 Nigeria
8.5.2.5.1 Nigeria Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.5.2.6 Rest of Africa
8.5.2.6.1 Rest of Africa Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.6 Latin America
8.6.1 Trends Analysis
8.6.2 Latin America Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Country (2020-2032) (USD Million)
8.6.3 Latin America Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.6.4 Brazil
8.6.4.1 Brazil Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.6.5 Argentina
8.6.5.1 Argentina Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.6.6 Colombia
8.6.6.1 Colombia Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
8.6.7 Rest of Latin America
8.6.7.1 Rest of Latin America Colorectal Cancer Therapeutics Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Million)
9. Company Profiles
9.1 Merck & Co., Inc.
9.1.1 Company Overview
9.1.2 Financial
9.1.3 Products/ Services Offered
9.1.4 SWOT Analysis
9.2 F. Hoffmann-La Roche Ltd.
9.2.1 Company Overview
9.2.2 Financial
9.2.3 Products/ Services Offered
9.2.4 SWOT Analysis
9.3 Bristol-Myers Squibb Company
9.3.1 Company Overview
9.3.2 Financial
9.3.3 Products/ Services Offered
9.3.4 SWOT Analysis
9.4 Amgen, Inc.
9.4.1 Company Overview
9.4.2 Financial
9.4.3 Products/ Services Offered
9.4.4 SWOT Analysis
9.5 Bayer AG
9.5.1 Company Overview
9.5.2 Financial
9.5.3 Products/ Services Offered
9.5.4 SWOT Analysis
9.6 Sanofi
9.6.1 Company Overview
9.6.2 Financial
9.6.3 Products/ Services Offered
9.6.4 SWOT Analysis
9.7 Genentech, Inc.
9.7.1 Company Overview
9.7.2 Financial
9.7.3 Products/ Services Offered
9.7.4 SWOT Analysis
9.8 Eli Lilly and Company
9.8.1 Company Overview
9.8.2 Financial
9.8.3 Products/ Services Offered
9.8.4 SWOT Analysis
9.9 Teva Pharmaceutical Industries Ltd.
9.9.1 Company Overview
9.9.2 Financial
9.9.3 Products/ Services Offered
9.9.4 SWOT Analysis
9.10 Pfizer Inc.
9.10.1 Company Overview
9.10.2 Financial
9.10.3 Products/ Services Offered
9.10.4 SWOT Analysis
10. Use Cases and Best Practices
11. Conclusion
An accurate research report requires proper strategizing as well as implementation. There are multiple factors involved in the completion of good and accurate research report and selecting the best methodology to compete the research is the toughest part. Since the research reports we provide play a crucial role in any company’s decision-making process, therefore we at SNS Insider always believe that we should choose the best method which gives us results closer to reality. This allows us to reach at a stage wherein we can provide our clients best and accurate investment to output ratio.
Each report that we prepare takes a timeframe of 350-400 business hours for production. Starting from the selection of titles through a couple of in-depth brain storming session to the final QC process before uploading our titles on our website we dedicate around 350 working hours. The titles are selected based on their current market cap and the foreseen CAGR and growth.
The 5 steps process:
Step 1: Secondary Research:
Secondary Research or Desk Research is as the name suggests is a research process wherein, we collect data through the readily available information. In this process we use various paid and unpaid databases which our team has access to and gather data through the same. This includes examining of listed companies’ annual reports, Journals, SEC filling etc. Apart from this our team has access to various associations across the globe across different industries. Lastly, we have exchange relationships with various university as well as individual libraries.

Step 2: Primary Research
When we talk about primary research, it is a type of study in which the researchers collect relevant data samples directly, rather than relying on previously collected data. This type of research is focused on gaining content specific facts that can be sued to solve specific problems. Since the collected data is fresh and first hand therefore it makes the study more accurate and genuine.
We at SNS Insider have divided Primary Research into 2 parts.
Part 1 wherein we interview the KOLs of major players as well as the upcoming ones across various geographic regions. This allows us to have their view over the market scenario and acts as an important tool to come closer to the accurate market numbers. As many as 45 paid and unpaid primary interviews are taken from both the demand and supply side of the industry to make sure we land at an accurate judgement and analysis of the market.
This step involves the triangulation of data wherein our team analyses the interview transcripts, online survey responses and observation of on filed participants. The below mentioned chart should give a better understanding of the part 1 of the primary interview.

Part 2: In this part of primary research the data collected via secondary research and the part 1 of the primary research is validated with the interviews from individual consultants and subject matter experts.
Consultants are those set of people who have at least 12 years of experience and expertise within the industry whereas Subject Matter Experts are those with at least 15 years of experience behind their back within the same space. The data with the help of two main processes i.e., FGDs (Focused Group Discussions) and IDs (Individual Discussions). This gives us a 3rd party nonbiased primary view of the market scenario making it a more dependable one while collation of the data pointers.
Step 3: Data Bank Validation
Once all the information is collected via primary and secondary sources, we run that information for data validation. At our intelligence centre our research heads track a lot of information related to the market which includes the quarterly reports, the daily stock prices, and other relevant information. Our data bank server gets updated every fortnight and that is how the information which we collected using our primary and secondary information is revalidated in real time.

Step 4: QA/QC Process
After all the data collection and validation our team does a final level of quality check and quality assurance to get rid of any unwanted or undesired mistakes. This might include but not limited to getting rid of the any typos, duplication of numbers or missing of any important information. The people involved in this process include technical content writers, research heads and graphics people. Once this process is completed the title gets uploader on our platform for our clients to read it.
Step 5: Final QC/QA Process:
This is the last process and comes when the client has ordered the study. In this process a final QA/QC is done before the study is emailed to the client. Since we believe in giving our clients a good experience of our research studies, therefore, to make sure that we do not lack at our end in any way humanly possible we do a final round of quality check and then dispatch the study to the client.
Key Segmentation
By Drug Class
Immunotherapy
Chemotherapy
Others
Request for Segment Customization as per your Business Requirement: Segment Customization Request
Regional Coverage:
North America
US
Canada
Mexico
Europe
Eastern Europe
Poland
Romania
Hungary
Turkey
Rest of Eastern Europe
Western Europe
Germany
France
UK
Italy
Spain
Netherlands
Switzerland
Austria
Rest of Western Europe
Asia Pacific
China
India
Japan
South Korea
Vietnam
Singapore
Australia
Rest of Asia Pacific
Middle East & Africa
Middle East
UAE
Egypt
Saudi Arabia
Qatar
Rest of the Middle East
Africa
Nigeria
South Africa
Rest of Africa
Latin America
Brazil
Argentina
Colombia
Rest of Latin America
Request for Country Level Research Report: Country Level Customization Request
Available Customization
With the given market data, SNS Insider offers customization as per the company’s specific needs. The following customization options are available for the report:
Product Analysis
Criss-Cross segment analysis (e.g. Product X Application)
Product Matrix which gives a detailed comparison of the product portfolio of each company
Geographic Analysis
Additional countries in any of the regions
Company Information
Detailed analysis and profiling of additional market players (Up to five)
The Peripheral Neuropathy Market valued USD 1.48 billion in 2023, estimated to surpass USD 2.38 billion by 2032 with a compound annual growth rate of 5.46% over the forecast period 2024-2032.
The BOTOX market size was valued at USD 5.58 billion in 2023 and is projected to reach USD 13.74 billion by 2032, growing at a CAGR of 10.55% from 2024-2032.
The Respiratory Devices Market was valued at USD 22.62 billion in 2023 and is projected to reach USD 46.10 billion by 2032, growing at a robust CAGR of 8.27 % during the forecast period of 2024-2032.
Natural Language Processing (NLP) in Healthcare and Life Sciences Market was valued at USD 4.94 billion in 2023 and is expected to reach USD 62.7 billion by 2032, growing at a CAGR of 32.6% over the forecast period 2024-2032.
The Artificial Intelligence in Drug Discovery Market Size was valued at USD 1.48 billion in 2023 and is expected to reach USD 15.50 billion by 2032 with a growing CAGR of 29.89%.
The Functional Endoscopic Sinus Surgery Market Size was valued at USD 1.01 Billion in 2023, and is expected to reach USD 1.91 Billion by 2032, and grow at a CAGR of 7.66% over the Forecast Period of 2024-2032.
Hi! Click one of our member below to chat on Phone