Get More Information on Cell Therapy Market- Request Sample Report
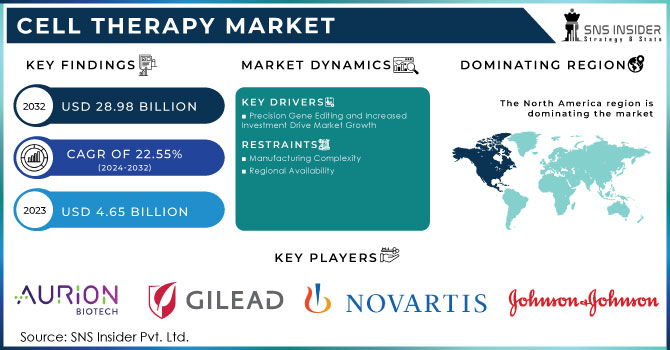
The Cell Therapy Market was valued at USD 4.65 Billion in 2023 and is expected to reach USD 28.98 billion by 2032 and grow at a CAGR of 22.55% over the forecast period 2024-2032.
Expanding Understanding of Cell-Based Treatments Drives Innovation
The cell therapy market is witnessing exponential growth, driven by cutting-edge technological advancements, robust funding, and a deeper understanding of the therapeutic potential of cell-based treatments. Companies are heavily investing in research and development to innovate and bring forward novel cell therapies targeting a diverse range of diseases.
Technological Advancements and Funding Propel the Cell Therapy Market
Technological advancements, particularly the development of CRISPR-Cas9 gene editing tools, are enabling the creation of more precise and effective cell therapies. This technology, combined with increased funding from government grants, venture capital investments, and strategic partnerships, is accelerating research, development, and clinical trials. Moreover, the growing prevalence of chronic diseases and the limitations of traditional treatments are increasing the demand for cell-based therapies. For instance, Achilles Therapeutics secured a USD 4.2 million grant from Horizon Europe, the EU's leading funding program for research and innovation, in July 2022, to advance personalized therapies.
Challenges in the Cell Therapy Market
Despite rapid growth, the cell therapy market faces significant challenges that could impact its trajectory. Complex and specialized manufacturing processes are required for cell therapies, making production costly and challenging. Additionally, navigating the regulatory landscape is often time-consuming and expensive, as companies must adhere to stringent safety and efficacy regulations. Securing reimbursement from healthcare payers for these therapies, particularly for high-cost treatments, remains a hurdle. These challenges may pose obstacles to the widespread adoption and commercialization of cell therapies.
Key Trends Shaping the Cell Therapy Market
The future of the cell therapy market is being shaped by several key trends. Personalized medicine is gaining traction as cell therapies offer the potential for highly tailored treatments based on individual genetic profiles, leading to improved outcomes and reduced side effects. Stem cell therapy is also gaining momentum for its potential to treat a broad spectrum of diseases, including autoimmune and metabolic disorders. Gene editing technologies like CRISPR-Cas9 are driving the development of more precise and effective cell therapies. Additionally, industry consolidation through mergers and acquisitions is becoming more common as companies aim to expand their product pipelines, geographic reach, and manufacturing capabilities. For example, in May 2022, Sernova and Evotec collaborated on the development of iPSC-based cell therapies for patients with insulin-dependent diabetes.
Growth Outlook for the Cell Therapy Market
The cell therapy market is expected to continue its upward growth trajectory, fueled by technological advancements, increased funding, and rising demand. As more cell therapy products receive regulatory approvals and become available to patients, the market is poised to make a significant impact on healthcare. However, overcoming challenges related to manufacturing, regulation, and reimbursement will be essential for sustained growth.
Market Dynamics
Drivers
Precision Gene Editing and Increased Investment Drive Market Growth
Precision gene editing technologies, such as CRISPR-Cas9, are enabling scientists to make precise modifications to genes, leading to the creation of more effective and personalized cell therapies. This has spurred increased interest and investment in the field, with companies and research institutions focusing on developing innovative treatments for a wide range of diseases. The combination of precision gene editing and increased investment is expected to drive significant growth in the cell therapy market in the coming years.
Restraints
Manufacturing Complexity
The production of cell therapies often involves complex and specialized manufacturing processes, which can be challenging and costly.
Cell therapies can be expensive, limiting their accessibility to patients.
Regional Availability
Cell therapies may not be available in all regions or healthcare settings.
Limited Clinical Data
Uncertainty Regarding Long-Term Outcomes
High Costs
By Type
Autologous Therapy Segment
Dominating the market with a 91.2% share in 2023, this segment's growth is driven by the widespread adoption of CAR-T therapies, which have shown promising results in treating various cancers and genetic disorders. FDA approvals are rapidly expanding their adoption. For example, in February 2022, the FDA approved ciltacabtagene autoleucel (Carvykti) for adult patients with multiple myeloma who have relapsed or are refractory to other treatments.
Allogeneic Cell Therapy Segment
Expected to experience significant growth from 2024 to 2032, this segment is gaining traction due to its increasing adoption in developing innovative therapeutic regimens. Currently, there are 542 active allogeneic CAR-T agents in the global pipeline, many showing promising outcomes. For instance, Adaptimmune Ltd. is collaborating with Genentech to explore iPSC-derived allogeneic therapies for creating T-cells with enhanced proliferation capabilities compared to mature T-cells.
By Therapeutic Area
Oncology Segment
The oncology segment led the market with the largest revenue 29.8% share in 2023. CAR T-cells targeting CD19 have demonstrated high rates of complete and long-lasting remissions in patients with acute lymphocytic leukemia (ALL). The increasing FDA approval of novel therapies is expected to create growth opportunities. For instance, in October 2021, the FDA approved brexucabtagene autoleucel (Tecartus), a CAR T therapy for adults with B-cell precursor ALL who have relapsed or are refractory to prior treatment. This approval marked brexucabtagene as the first CAR T treatment for adults with ALL.
Musculoskeletal Disorders Segment
Anticipated to see significant market expansion, ongoing research is focused on developing technologies to regenerate or repair damaged musculoskeletal tissues. Researchers are studying clinically applicable cell types for musculoskeletal tissue regeneration therapies, as well as exploring the direct application of engineered or native skeletal progenitor cells to stimulate tissue repair and revitalize musculoskeletal tissues. These factors are expected to drive the growth of this segment.
Regional Analysis
North America
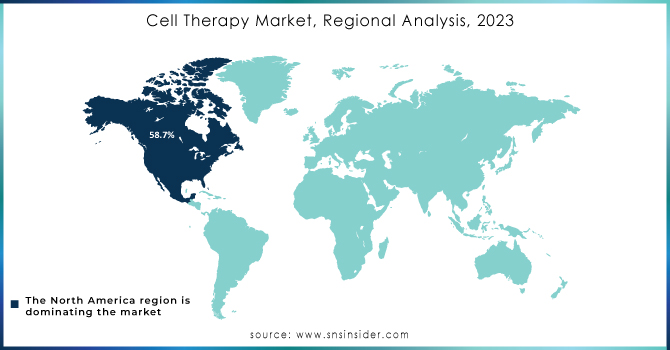
The North American market dominated the cell therapy industry with a revenue share of 58.7% in 2023, attributed to collaborative research efforts between research institutes and pharmaceutical giants in the region. Numerous collaborations have led to significant advancements in cell therapy. For example, in June 2022, Immatics partnered with Bristol Myers Squibb to develop Gamma Delta Allogeneic Cell Therapy Programs. Government funding has also been a crucial driver of market growth in the U.S. In January 2022, Cellino Biotech raised USD 80 million in a Series A funding round to expand access to stem cell-derived therapies and develop the first independent human cell foundry by 2025.
Asia-Pacific
The Asia-Pacific region is projected to experience substantial growth in the cell therapy market during the forecast period. Increased awareness of novel therapies, growing investments, and favorable government policies are key factors expected to accelerate market expansion. For instance, in June 2022, Tessa Therapeutics Ltd. raised USD 126 million to fund the development of next-generation cancer therapies. The South Korean market is also anticipated to exhibit strong growth due to strategic initiatives by local and international companies. In August 2022, Panacea Biotech announced plans to use Natural Killer (NK) cells, brown Adipose-Derived Stem Cells (ADSC), and exosomes for treating COVID-19 infection.
Need any customization research on Cell Therapy Market - Enquiry Now
JCR Pharmaceuticals Co., Ltd.
Novartis AG
Nkarta, Inc.
Bristol-Myers Squibb Company
JW Therapeutics
S. BIOMEDICS
Atara Biotherapeutics
Holostem Terapie Avanzate S.r.l
Anterogen Co., Ltd., and others
Recent Developments
Novartis: In January 2024, Novartis announced positive results from a Phase III clinical trial of Kymriah (tisagenlecleucel) for the treatment of relapsed/refractory diffuse large B-cell lymphoma.
Gilead Sciences: In February 2024, Gilead Sciences initiated a Phase III clinical trial for its CAR T cell therapy, Yescarta, to treat patients with relapsed/refractory follicular lymphoma.
Bluebird Bio: In March 2024, Bluebird Bio announced positive results from a Phase III clinical trial of its gene therapy, LentiGlobin, for the treatment of sickle cell disease.
CRISPR Therapeutics: In April 2024, CRISPR Therapeutics and Vertex Pharmaceuticals initiated a Phase III clinical trial for their CRISPR-based gene therapy, CTX001, to treat sickle cell disease and beta-thalassemia.
Celgene: In May 2024, Celgene, a Bristol Myers Squibb company, initiated a Phase III clinical trial for its CAR T cell therapy, JCAR015, to treat patients with relapsed/refractory multiple myeloma.
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | US$ 4.65 Billion |
| Market Size by 2032 | US$ 28.98 billion |
| CAGR | CAGR of 22.55% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | •By Type [Allogeneic Therapies (Stem Cell Therapies, Non-Stem Cell Therapies), Autologous Therapies (Stem Cell Therapies, Non-Stem Cell Therapies)] •By Therapeutic Area [Oncology, Cardiovascular Disease (CVD), Musculoskeletal Disorders, Dermatology, Others] |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | Aurion Biotech, Gilead Sciences, Inc., JCR Pharmaceuticals Co., Ltd., Novartis AG, Johnson & Johnson Services, Inc, Nkarta, Inc., Bristol-Myers Squibb Company, MEDIPOST, JW Therapeutics, S. BIOMEDICS, Atara Biotherapeutics, Holostem Terapie Avanzate S.r.l, Anterogen Co., Ltd., and others. |
| Key Drivers | • Precision Gene Editing and Increased Investment Drive Market Growth |
| Restraints | •Manufacturing Complexity • Regional Availability • Limited Clinical Data • Uncertainty Regarding Long-Term Outcomes • High Costs |
Ans: The estimated compound annual growth rate is 22.55% during the forecast period for the Cell Therapy market.
Ans: The projected market value of the Cell Therapy market is estimated at USD 4.65 Billion in 2023 and is expected to reach USD 28.98 billion by 2032.
Ans: Precision gene editing and increased investment drive cell therapy market growth.
Ans: Manufacturing Complexity is one of the restraints on the cell therapy market growth.
Ans: North America is the dominating region with a 58.7% market share in the Cell Therapy market.
Table of Contents
1. Introduction
1.1 Market Definition
1.2 Scope (Inclusion and Exclusions)
1.3 Research Assumptions
2. Executive Summary
2.1 Market Overview
2.2 Regional Synopsis
2.3 Competitive Summary
3. Research Methodology
3.1 Top-Down Approach
3.2 Bottom-up Approach
3.3. Data Validation
3.4 Primary Interviews
4. Market Dynamics Impact Analysis
4.1 Market Driving Factors Analysis
4.1.2 Drivers
4.1.2 Restraints
4.1.3 Opportunities
4.1.4 Challenges
4.2 PESTLE Analysis
4.3 Porter’s Five Forces Model
5. Statistical Insights and Trends Reporting
5.1 Incidence and Prevalence (2023)
5.2 Prescription Trends, (2023), by Region
5.3 Drug Volume: Production and usage volumes of pharmaceuticals.
5.4 Healthcare Spending: Expenditure data by government, insurers, and out-of-pocket by patients.
6. Competitive Landscape
6.1 List of Major Companies, By Region
6.2 Market Share Analysis, By Region
6.3 Product Benchmarking
6.3.1 Product specifications and features
6.3.2 Pricing
6.4 Strategic Initiatives
6.4.1 Marketing and promotional activities
6.4.2 Distribution and Supply Chain Strategies
6.4.3 Expansion plans and new product launches
6.4.4 Strategic partnerships and collaborations
6.5 Technological Advancements
6.6 Market Positioning and Branding
7. Cell Therapy Market Segmentation, by Type
7.1 Chapter Overview
7.2 Allogeneic Therapies
7.2.1 Allogeneic Therapies Market Trends Analysis (2020-2032)
7.2.2 Allogeneic Therapies Market Size Estimates and Forecasts to 2032 (USD Billion)
7.2.3 Stem Cell Therapies
7.2.3.1 Stem Cell Therapies Market Trends Analysis (2020-2032)
7.2.3.2 Stem Cell Therapies Market Size Estimates and Forecasts to 2032 (USD Billion)
7.2.4 Non-Stem Cell Therapies
7.2.4.1 Non-Stem Cell Therapies Market Trends Analysis (2020-2032)
7.2.4.2 Non-Stem Cell Therapies Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3 Autologous Therapies
7.3.1 Autologous Therapies Market Trends Analysis (2020-2032)
7.3.2 Autologous Therapies Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.3 Stem Cell Therapies
7.3.3.1 Stem Cell Therapies Market Trends Analysis (2020-2032)
7.3.3.2 Stem Cell Therapies Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.4 Non-Stem Cell Therapies
7.3.4.1 Non-Stem Cell Therapies Market Trends Analysis (2020-2032)
7.3.4.2 Non-Stem Cell Therapies Market Size Estimates and Forecasts to 2032 (USD Billion)
8. Cell Therapy Market Segmentation, by Therapeutic Area
8.1 Chapter Overview
8.2 Oncology
8.2.1 Oncology Market Trends Analysis (2020-2032)
8.2.2 Oncology Market Size Estimates and Forecasts to 2032 (USD Billion)
8.3 cardiovascular disease (CVD)
8.3.1 Cardiovascular Disease (CVD) Market Trends Analysis (2020-2032)
8.3.2 Cardiovascular Disease (CVD) Market Size Estimates and Forecasts to 2032 (USD Billion)
8.4 Musculoskeletal Disorders
8.4.1 Musculoskeletal Disorders Market Trends Analysis (2020-2032)
8.4.2 Musculoskeletal Disorders Market Size Estimates and Forecasts to 2032 (USD Billion)
8.5 Dermatology
8.5.1 Dermatology Market Trends Analysis (2020-2032)
8.5.2 Dermatology Market Size Estimates and Forecasts to 2032 (USD Billion)
8.6 Other
8.6.1 Other Market Trends Analysis (2020-2032)
8.6.2 Other Market Size Estimates and Forecasts to 2032 (USD Billion)
9. Regional Analysis
9.1 Chapter Overview
9.2 North America
9.2.1 Trends Analysis
9.2.2 North America Cell Therapy Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.2.3 North America Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.2.4 North America Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.2.5 USA
9.2.5.1 USA Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.2.5.2 USA Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.2.6 Canada
9.2.6.1 Canada Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.2.6.2 Canada Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.2.7 Mexico
9.2.7.1 Mexico Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.2.7.2 Mexico Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.3 Europe
9.3.1 Eastern Europe
9.3.1.1 Trends Analysis
9.3.1.2 Eastern Europe Cell Therapy Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.3.1.3 Eastern Europe Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.3.1.4 Eastern Europe Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.3.1.5 Poland
9.3.1.5.1 Poland Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.3.1.5.2 Poland Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.3.1.6 Romania
9.3.1.6.1 Romania Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.3.1.6.2 Romania Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.3.1.7 Hungary
9.3.1.7.1 Hungary Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.3.1.7.2 Hungary Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.3.1.8 Turkey
9.3.1.8.1 Turkey Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.3.1.8.2 Turkey Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.3.1.9 Rest of Eastern Europe
9.3.1.9.1 Rest of Eastern Europe Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.3.1.9.2 Rest of Eastern Europe Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.3.2 Western Europe
9.3.2.1 Trends Analysis
9.3.2.2 Western Europe Cell Therapy Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.3.2.3 Western Europe Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.3.2.4 Western Europe Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.3.2.5 Germany
9.3.2.5.1 Germany Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.3.2.5.2 Germany Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.3.2.6 France
9.3.2.6.1 France Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.3.2.6.2 France Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.3.2.7 UK
9.3.2.7.1 UK Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.3.2.7.2 UK Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.3.2.8 Italy
9.3.2.8.1 Italy Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.3.2.8.2 Italy Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.3.2.9 Spain
9.3.2.9.1 Spain Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.3.2.9.2 Spain Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.3.2.10 Netherlands
9.3.2.10.1 Netherlands Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.3.2.10.2 Netherlands Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.3.2.11 Switzerland
9.3.2.11.1 Switzerland Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.3.2.11.2 Switzerland Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.3.2.12 Austria
9.3.2.12.1 Austria Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.3.2.12.2 Austria Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.3.2.13 Rest of Western Europe
9.3.2.13.1 Rest of Western Europe Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.3.2.13.2 Rest of Western Europe Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.4 Asia Pacific
9.4.1 Trends Analysis
9.4.2 Asia Pacific Cell Therapy Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.4.3 Asia Pacific Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.4.4 Asia Pacific Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.4.5 China
9.4.5.1 China Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.4.5.2 China Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.4.6 India
9.4.5.1 India Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.4.5.2 India Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.4.5 Japan
9.4.5.1 Japan Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.4.5.2 Japan Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.4.6 South Korea
9.4.6.1 South Korea Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.4.6.2 South Korea Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.4.7 Vietnam
9.4.7.1 Vietnam Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.2.7.2 Vietnam Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.4.8 Singapore
9.4.8.1 Singapore Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.4.8.2 Singapore Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.4.9 Australia
9.4.9.1 Australia Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.4.9.2 Australia Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.4.10 Rest of Asia Pacific
9.4.10.1 Rest of Asia Pacific Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.4.10.2 Rest of Asia Pacific Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.5 Middle East and Africa
9.5.1 Middle East
9.5.1.1 Trends Analysis
9.5.1.2 Middle East Cell Therapy Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.5.1.3 Middle East Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.5.1.4 Middle East Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.5.1.5 UAE
9.5.1.5.1 UAE Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.5.1.5.2 UAE Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.5.1.6 Egypt
9.5.1.6.1 Egypt Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.5.1.6.2 Egypt Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.5.1.7 Saudi Arabia
9.5.1.7.1 Saudi Arabia Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.5.1.7.2 Saudi Arabia Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.5.1.8 Qatar
9.5.1.8.1 Qatar Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.5.1.8.2 Qatar Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.5.1.9 Rest of Middle East
9.5.1.9.1 Rest of Middle East Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.5.1.9.2 Rest of Middle East Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.5.2 Africa
9.5.2.1 Trends Analysis
9.5.2.2 Africa Cell Therapy Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.5.2.3 Africa Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.5.2.4 Africa Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.5.2.5 South Africa
9.5.2.5.1 South Africa Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.5.2.5.2 South Africa Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.5.2.6 Nigeria
9.5.2.6.1 Nigeria Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.5.2.6.2 Nigeria Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.5.2.7 Rest of Africa
9.5.2.7.1 Rest of Africa Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.5.2.7.2 Rest of Africa Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.6 Latin America
9.6.1 Trends Analysis
9.6.2 Latin America Cell Therapy Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.6.3 Latin America Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.6.4 Latin America Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.6.5 Brazil
9.6.5.1 Brazil Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.6.5.2 Brazil Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.6.6 Argentina
9.6.6.1 Argentina Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.6.6.2 Argentina Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.6.7 Colombia
9.6.7.1 Colombia Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.6.7.2 Colombia Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
9.6.8 Rest of Latin America
9.6.8.1 Rest of Latin America Cell Therapy Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.6.8.2 Rest of Latin America Cell Therapy Market Estimates and Forecasts, by Therapeutic Area (2020-2032) (USD Billion)
10. Company Profiles
10.1 Aurion Biotech
10.1.1 Company Overview
10.1.2 Financial
10.1.3 Products/ Services Offered
110.1.4 SWOT Analysis
10.2 Gilead Sciences, Inc.
10.2.1 Company Overview
10.2.2 Financial
10.2.3 Products/ Services Offered
10.2.4 SWOT Analysis
10.3 JCR Pharmaceuticals Co., Ltd.
10.3.1 Company Overview
10.3.2 Financial
10.3.3 Products/ Services Offered
10.3.4 SWOT Analysis
10.4 Novartis AG
10.4.1 Company Overview
10.4.2 Financial
10.4.3 Products/ Services Offered
10.4.4 SWOT Analysis
10.5 Johnson & Johnson Services, Inc
10.5.1 Company Overview
10.5.2 Financial
10.5.3 Products/ Services Offered
10.5.4 SWOT Analysis
10.6 Nkarta, Inc.
10.6.1 Company Overview
10.6.2 Financial
10.6.3 Products/ Services Offered
10.6.4 SWOT Analysis
10.7 Bristol-Myers Squibb Company
10.7.1 Company Overview
10.7.2 Financial
10.7.3 Products/ Services Offered
10.7.4 SWOT Analysis
10.8 MEDIPOST
10.8.1 Company Overview
10.8.2 Financial
10.8.3 Products/ Services Offered
10.8.4 SWOT Analysis
10.9 JW Therapeutics
10.9.1 Company Overview
10.9.2 Financial
10.9.3 Products/ Services Offered
10.9.4 SWOT Analysis
10.10 S. BIOMEDICS
10.9.1 Company Overview
10.9.2 Financial
10.9.3 Products/ Services Offered
10.9.4 SWOT Analysis
11. Use Cases and Best Practices
12. Conclusion
An accurate research report requires proper strategizing as well as implementation. There are multiple factors involved in the completion of good and accurate research report and selecting the best methodology to compete the research is the toughest part. Since the research reports we provide play a crucial role in any company’s decision-making process, therefore we at SNS Insider always believe that we should choose the best method which gives us results closer to reality. This allows us to reach at a stage wherein we can provide our clients best and accurate investment to output ratio.
Each report that we prepare takes a timeframe of 350-400 business hours for production. Starting from the selection of titles through a couple of in-depth brain storming session to the final QC process before uploading our titles on our website we dedicate around 350 working hours. The titles are selected based on their current market cap and the foreseen CAGR and growth.
The 5 steps process:
Step 1: Secondary Research:
Secondary Research or Desk Research is as the name suggests is a research process wherein, we collect data through the readily available information. In this process we use various paid and unpaid databases which our team has access to and gather data through the same. This includes examining of listed companies’ annual reports, Journals, SEC filling etc. Apart from this our team has access to various associations across the globe across different industries. Lastly, we have exchange relationships with various university as well as individual libraries.

Step 2: Primary Research
When we talk about primary research, it is a type of study in which the researchers collect relevant data samples directly, rather than relying on previously collected data. This type of research is focused on gaining content specific facts that can be sued to solve specific problems. Since the collected data is fresh and first hand therefore it makes the study more accurate and genuine.
We at SNS Insider have divided Primary Research into 2 parts.
Part 1 wherein we interview the KOLs of major players as well as the upcoming ones across various geographic regions. This allows us to have their view over the market scenario and acts as an important tool to come closer to the accurate market numbers. As many as 45 paid and unpaid primary interviews are taken from both the demand and supply side of the industry to make sure we land at an accurate judgement and analysis of the market.
This step involves the triangulation of data wherein our team analyses the interview transcripts, online survey responses and observation of on filed participants. The below mentioned chart should give a better understanding of the part 1 of the primary interview.

Part 2: In this part of primary research the data collected via secondary research and the part 1 of the primary research is validated with the interviews from individual consultants and subject matter experts.
Consultants are those set of people who have at least 12 years of experience and expertise within the industry whereas Subject Matter Experts are those with at least 15 years of experience behind their back within the same space. The data with the help of two main processes i.e., FGDs (Focused Group Discussions) and IDs (Individual Discussions). This gives us a 3rd party nonbiased primary view of the market scenario making it a more dependable one while collation of the data pointers.
Step 3: Data Bank Validation
Once all the information is collected via primary and secondary sources, we run that information for data validation. At our intelligence centre our research heads track a lot of information related to the market which includes the quarterly reports, the daily stock prices, and other relevant information. Our data bank server gets updated every fortnight and that is how the information which we collected using our primary and secondary information is revalidated in real time.

Step 4: QA/QC Process
After all the data collection and validation our team does a final level of quality check and quality assurance to get rid of any unwanted or undesired mistakes. This might include but not limited to getting rid of the any typos, duplication of numbers or missing of any important information. The people involved in this process include technical content writers, research heads and graphics people. Once this process is completed the title gets uploader on our platform for our clients to read it.
Step 5: Final QC/QA Process:
This is the last process and comes when the client has ordered the study. In this process a final QA/QC is done before the study is emailed to the client. Since we believe in giving our clients a good experience of our research studies, therefore, to make sure that we do not lack at our end in any way humanly possible we do a final round of quality check and then dispatch the study to the client.
Key Segments:
By Type
Allogeneic Therapies
Stem Cell Therapies
Non-Stem Cell Therapies
Autologous Therapies
Stem Cell Therapies
Non-Stem Cell Therapies
By Therapeutic Area
Oncology
Cardiovascular Disease (CVD)
Musculoskeletal Disorders
Dermatology
Others
Request for Segment Customization as per your Business Requirement: Segment Customization Request
Regional Coverage:
North America
US
Canada
Mexico
Europe
Eastern Europe
Poland
Romania
Hungary
Turkey
Rest of Eastern Europe
Western Europe
Germany
France
UK
Italy
Spain
Netherlands
Switzerland
Austria
Rest of Western Europe
Asia Pacific
China
India
Japan
South Korea
Vietnam
Singapore
Australia
Rest of Asia Pacific
Middle East & Africa
Middle East
UAE
Egypt
Saudi Arabia
Qatar
Rest of the Middle East
Africa
Nigeria
South Africa
Rest of Africa
Latin America
Brazil
Argentina
Colombia
Rest of Latin America
Request for Country Level Research Report: Country Level Customization Request
Available Customization
With the given market data, SNS Insider offers customization as per the company’s specific needs. The following customization options are available for the report:
Product Analysis
Criss-Cross segment analysis (e.g. Product X Application)
Product Matrix which gives a detailed comparison of the product portfolio of each company
Geographic Analysis
Additional countries in any of the regions
Company Information
Detailed analysis and profiling of additional market players (Up to five)
The Breast Cancer Core Needle Biopsy Market was valued at USD 809.40 million in 2023 and is expected to reach USD 1357.70 million by 2032, growing at a CAGR of 8.32% from 2024-2032.
The Research Antibodies Market Size was valued at USD 1.4 billion in 2023 and expected to reach USD 2.3 billion by 2032 and grow at a CAGR of 5.5%.
The Image-guided Biopsy market size was USD 4.01 billion in 2023 and is expected to reach USD 6.98 billion by 2032 and grow at a CAGR of 6.35% over the forecast period of 2024-2032.
The Proteinase K Market was valued at USD 4.46 billion in 2023 and is projected to grow to USD 9.30 billion by 2032, with a CAGR of 8.47%.
The global 3D Cell Culture Market, valued at USD 1.4 billion in 2023 and projected to reach USD 4.0 billion by 2032, growing at a CAGR of 12.4% by 2032.
The Ureteroscope Market Size was valued at USD 1,056.2 Million in 2023 and is expected to reach USD 1,685.14 Million by 2032 and grow at a CAGR of 5.54% over the forecast period 2024-2032.
Hi! Click one of our member below to chat on Phone