Cardiac Safety Services Market Report Scope & Overview:
Get more information on Cardiac Safety Services Market - Request Free Sample Report
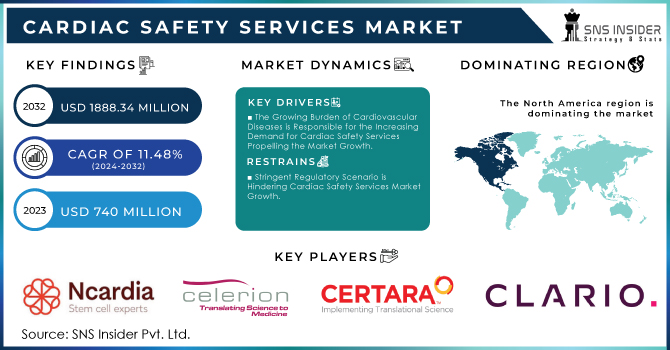
The Cardiac Safety Services Market Size was valued at USD 740 Million in 2023 and is expected to reach USD 1,888.34 Million by 2032 and grow at a CAGR of 11.48% over the forecast period 2024-2032. Growth in the cardiac safety services market is mainly received from % several key factors, which include various government statistics. In the first place, there is an increase in the incidence of cardiovascular diseases. CVDs are the number one cause of death globally: more people die annually from CVDs than from any other cause; an estimated 17.9 million percent, account for three-quarters of all deaths due to non-communicable diseases as per the World Health Organization. This distressing figure illustrates the necessity for comprehensive cardiac safety solutions that both clinical trials, as well as everyday healthcare, can utilize to keep track of and tackle risks at an early stage.
Moreover, the increase in clinical trials (especially those conducted by pharmaceutical and biopharmaceutical organizations) further boosts the cardiological safety services market. As of 2023, more than 420,000 clinical trials have been registered across the world and this number is increasing at a constant rate by the National Institutes of Health. The rising trend underscores the emerging demand for thorough cardiac safety evaluations to ensure patient centricity as well as compliance with regulatory norms. The enforcement of government regulations and some guidelines also influences the growth of this market sector. the drug approval process by regulatory agencies such as The USFDA or the European Medicines Agency requires comprehensive cardiac safety evaluation of drugs. The demand for these services is driven by a need to perform extensive cardiac safety testing by the FDA's guidance on the evaluation of the proarrhythmic potential of drugs.
Besides technological progress, the development of artificial intelligence in cardiac safety services extends its power to higher levels. Growth initiatives taken by the government to accelerate the words AI in healthcare are fuelling these trends such as the digital health innovation plan from the FDA, which prescribed modern cardiac monitoring technologies manufacturing and implementation. To conclude the demand for cardiac safety services market is driven by the increasing incidence of cardiovascular disorders, a rise in the number of clinical trials, stringent regulations, and technological advancements. This is further highlighted by government statistics and regulations that are focused on the improved patient safety and operations-based compliance required as a result of changes in healthcare.
MARKET DYNAMICS:
KEY DRIVERS:
The Growing Burden of Cardiovascular Diseases is Responsible for the Increasing Demand for Cardiac Safety Services Propelling the Market Growth.
Increased Focus on Developing Personalized Medicines is Boosting the Cardiac Safety Services Market Growth.
RESTRAINTS:
Stringent Regulatory Scenario is Hindering Cardiac Safety Services Market Growth.
High Cost of Cardiac Safety Evaluation Can Limit the Growth of Cardiac Safety Services.
OPPORTUNITY:
An increase in Biologics & biosimilar development is Offering a Lucrative Growth Opportunity for Cardiac Safety Services Procedures.
Growth in the Analysis and Development Investment in Pharma are Responsible for the Market Growth During Upcoming Years.
KEY MARKET SEGMENTATION:
By Type
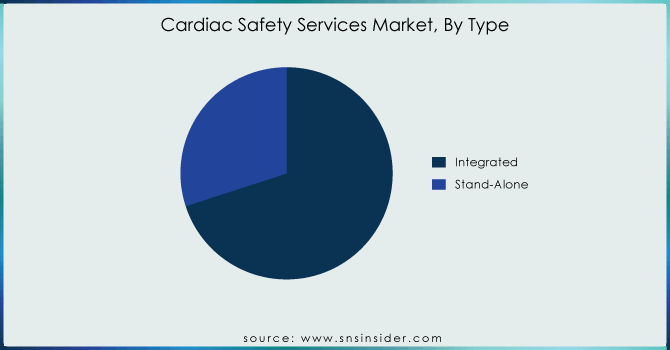
Integrated services held 70% as of the end of 2023. These are some of the primary reasons why integrated take-not-home services have been so successful. Data Integration: Firstly, integrated services combine all kinds of data to provide comprehensive integration and make sure that you have a consolidated whole dataset with everything related. By combining it with the data, integration will greatly enhance the quality of those data and help eliminate discrepancies/errors that often arise when you work on separate datasets. Further, integrated services support bio/pharma to execute clinical trials (which are pivotal through the pharmaceutical/biopharmaceutical ICO cycle) in improved and shorter lifecycle increments. These services speed up the process of clinical trials by simplifying data management and deployment procedures while saving costs. In the fast-moving world of drug development, rapid results are vital - meaning this efficiency is worth its weight in gold.
In addition, an integrated view of patient data by way of the comprehensive services saves us from missing out on what may be considered minor yet extremely important observations that could potentially influence cardiac safety handling during feature duration. This end-to-end process protects all data from trial design to final results thereby enhancing the reliability quotient of clinical outcomes as well as assuring compliance by making sure that detailed safety information is reliable and truly comprehensive. Hence, integrated services are the most sought-after option by a majority of players to streamline their cardiac safety monitoring attempts for serve as one of the leading producers in the cardiac safety solutions market.
Get Customized Report as per your Business Requirement - Request For Customized Report
By End-User
The Pharmaceutical and biopharmaceutical companies dominated the cardiac safety services market in 2023 with 45% of revenue. The companies undertake a plethora of clinical trials to manufacture new drugs and therapies. Every trial must include the stringent cardiac safety evaluations required for patient protection and regulatory compliance. As a result, there has been significant demand for cardiac safety services among these organizations. Additionally, companies working in the area of pharmaceutical and biopharmaceutical generally maintain a substantial resource pool for cardiac safety. This comes with expert teams, state-of-the-art tech, and advanced infrastructure for quick turnarounds in terms of efficient & effective cardiac safety monitoring. The accessibility of these assets empowers the share for different organizations to deal with heart well-being in contrast with other end-clients.
Regulatory compliance is another major factor that helps these incumbent magnates maintain their position. Both the FDA and EMA require comprehensive evaluations of drugs for cardiac safety as part of the drug approval process. However, a failure to comply with this strict law may lead to delays or rejection of drug approvals, amounting to large sums. Pharmaceutical & biopharma companies spend significantly on cardiac safety services to avoid such significant risks and ensure regulatory compliance. Moreover, rising patient safety awareness in these companies will continue to fuel the adoption of cardiac safety solutions that are comprehensive and seamlessly integrated. Patient safety is paramount throughout the clinical trial process and integrated cardiac safety services take a global, customized approach to monitoring and evaluating cardiac risk. This emphasis on patient safety not only leads to more successful trial results but also makes these companies recognized as pioneers in safe and reliable drug development.
REGIONAL ANALYSIS:
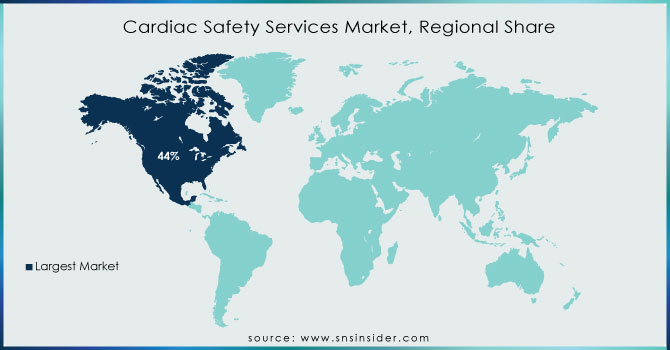
North America holds 44% in 2023 due to an increasing geriatric population and higher incidence rate for cardiovascular diseases. There were nearly 805,000 estimated heart attacks in the United States each year (605,000 new or first and 200,000 recurrent events) as of October 2020 according to updated information last published by the CDC. In addition, a growing geriatric population in the area is also anticipated to grow cardiovascular diseases hence adopting cardiac safety services. For example, published figures from StatsCan in July 2022 estimate that there are now approximately 7,330,605 Canadians who are aged 65 and this corresponds with a share of 20 percent of the entire population.
Moreover, significant contributions by the top regional market players are also likely to drive an increase in the revenue of the industry. For example, ERT and Bioclinica merged under the banner of UIW Clario in November 2021. The deal brings together Bioclinica's clinical trial imaging, eClinical software, and drug safety solutions with ERT's expertise in electronic Clinical Outcome Assessments (eCOA), cardiac safety, and respiratory health.
KEY PLAYERS:
The key market players include Ncardia AG, Celerion, Certara, Clario, Richmond Pharmacology, Koninklijke Philips N.V.(BioTelemetry), Laboratory Corporation of America Holdings, Medpace, Biotrial, PhysioStim & other players.
RECENT DEVELOPMENTS
In August 2022, FIFPRO and IDOVEN company were involved in a partnership to improve player health prevention from sudden cardiac arrest or any other heart risks.
In MAY 2022, Novocardia announced the launch of an edge program that has incorporated individualized health coaching paired with remote patient monitoring for heart failure patients aimed at enhancing their quality of life and reducing hospitalization rate.
| Report Attributes | Details |
| Market Size in 2023 |
US$ 740 Million |
| Market Size by 2032 |
US$ 1,888.34 Million |
| CAGR |
CAGR of 11.48% From 2024 to 2032 |
| Base Year |
2023 |
| Forecast Period |
2024-2032 |
| Historical Data |
2020-2022 |
| Report Scope & Coverage |
Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments |
•By Type (Integrated & Stand-alone) |
| Regional Analysis/Coverage |
North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles |
Ncardia AG, Celerion, Certara, Clario, Richmond Pharmacology, Koninklijke Philips N.V.(BioTelemetry), Laboratory Corporation of America Holdings, Medpace, Biotrial, PhysioStim & other players |
| Key Drivers |
•The Growing Burden of Cardiovascular Diseases is Responsible for the Increasing Demand for Cardiac Safety Services Propelling the Market Growth. |
| RESTRAINTS |
•Stringent Regulatory Scenario is Hindering Cardiac Safety Services Market Growth. |
Ans: The U.S. led the Cardiac Safety Services in the North American region with the highest revenue share in 2023.
Ans: Factors such as the stringent regulatory scenario as well as the high cost of cardiac safety evaluation can limit the growth of cardiac safety services.
Ans: Integrated Type will increase in the Cardiac Safety Services Market from 2024-2032.
Ans: The expected CAGR of the Global Cardiac Safety Services Market during the forecast period is 11.48%.
Ans: The Cardiac Safety Services Market was valued at USD 740 Million in 2023.
TABLE OF CONTENTS
1. Introduction
1.1 Market Definition
1.2 Scope
1.3 Research Assumptions
2. Industry Flowchart
3. Research Methodology
4. Market Dynamics
4.1 Drivers
4.2 Restraints
4.3 Opportunities
4.4 Challenges
5. Porter’s 5 Forces Model
6. Pest Analysis
7. Cardiac Safety Services Market Segmentation, By Type
7.1 Introduction
7.2 Integrated
7.3 Stand-alone
8. Cardiac Safety Services Market Segmentation, By Service
8.1 Introduction
8.2 ECG/Holter Measurement
8.3 Blood Pressure Measurement
8.4 Cardiovascular Imaging
8.5 Thorough QT Studies
8.6 Others
9. Cardiac Safety Services Market Segmentation, By End-User
9.1 Introduction
9.2 Pharmaceutical and Biopharmaceutical Companies
9.3 Contract Research Organizations
9.4 Others
10. Regional Analysis
10.1 Introduction
10.2 North America
10.2.1 Trend Analysis
10.2.2 North America Cardiac Safety Services Market by Country
10.2.3 North America Cardiac Safety Services Market By Type
10.2.4 North America Cardiac Safety Services Market By Service
10.2.5 North America Cardiac Safety Services Market By End-User
10.2.6 USA
10.2.6.1 USA Cardiac Safety Services Market By Type
10.2.6.2 USA Cardiac Safety Services Market By Service
10.2.6.3 USA Cardiac Safety Services Market By End-User
10.2.7 Canada
10.2.7.1 Canada Cardiac Safety Services Market By Type
10.2.7.2 Canada Cardiac Safety Services Market By Service
10.2.7.3 Canada Cardiac Safety Services Market By End-User
10.2.8 Mexico
10.2.8.1 Mexico Cardiac Safety Services Market By Type
10.2.8.2 Mexico Cardiac Safety Services Market By Service
10.2.8.3 Mexico Cardiac Safety Services Market By End-User
10.3 Europe
10.3.1 Trend Analysis
10.3.2 Eastern Europe
10.3.2.1 Eastern Europe Cardiac Safety Services Market by Country
10.3.2.2 Eastern Europe Cardiac Safety Services Market By Type
10.3.2.3 Eastern Europe Cardiac Safety Services Market By Service
10.3.2.4 Eastern Europe Cardiac Safety Services Market By End-User
10.3.2.5 Poland
10.3.2.5.1 Poland Cardiac Safety Services Market By Type
10.3.2.5.2 Poland Cardiac Safety Services Market By Service
10.3.2.5.3 Poland Cardiac Safety Services Market By End-User
10.3.2.6 Romania
10.3.2.6.1 Romania Cardiac Safety Services Market By Type
10.3.2.6.2 Romania Cardiac Safety Services Market By Service
10.3.2.6.4 Romania Cardiac Safety Services Market By End-User
10.3.2.7 Hungary
10.3.2.7.1 Hungary Cardiac Safety Services Market By Type
10.3.2.7.2 Hungary Cardiac Safety Services Market By Service
10.3.2.7.3 Hungary Cardiac Safety Services Market By End-User
10.3.2.8 Turkey
10.3.2.8.1 Turkey Cardiac Safety Services Market By Type
10.3.2.8.2 Turkey Cardiac Safety Services Market By Service
10.3.2.8.3 Turkey Cardiac Safety Services Market By End-User
10.3.2.9 Rest of Eastern Europe
10.3.2.9.1 Rest of Eastern Europe Cardiac Safety Services Market By Type
10.3.2.9.2 Rest of Eastern Europe Cardiac Safety Services Market By Service
10.3.2.9.3 Rest of Eastern Europe Cardiac Safety Services Market By End-User
10.3.3 Western Europe
10.3.3.1 Western Europe Cardiac Safety Services Market by Country
10.3.3.2 Western Europe Cardiac Safety Services Market By Type
10.3.3.3 Western Europe Cardiac Safety Services Market By Service
10.3.3.4 Western Europe Cardiac Safety Services Market By End-User
10.3.3.5 Germany
10.3.3.5.1 Germany Cardiac Safety Services Market By Type
10.3.3.5.2 Germany Cardiac Safety Services Market By Service
10.3.3.5.3 Germany Cardiac Safety Services Market By End-User
10.3.3.6 France
10.3.3.6.1 France Cardiac Safety Services Market By Type
10.3.3.6.2 France Cardiac Safety Services Market By Service
10.3.3.6.3 France Cardiac Safety Services Market By End-User
10.3.3.7 UK
10.3.3.7.1 UK Cardiac Safety Services Market By Type
10.3.3.7.2 UK Cardiac Safety Services Market By Service
10.3.3.7.3 UK Cardiac Safety Services Market By End-User
10.3.3.8 Italy
10.3.3.8.1 Italy Cardiac Safety Services Market By Type
10.3.3.8.2 Italy Cardiac Safety Services Market By Service
10.3.3.8.3 Italy Cardiac Safety Services Market By End-User
10.3.3.9 Spain
10.3.3.9.1 Spain Cardiac Safety Services Market By Type
10.3.3.9.2 Spain Cardiac Safety Services Market By Service
10.3.3.9.3 Spain Cardiac Safety Services Market By End-User
10.3.3.10 Netherlands
10.3.3.10.1 Netherlands Cardiac Safety Services Market By Type
10.3.3.10.2 Netherlands Cardiac Safety Services Market By Service
10.3.3.10.3 Netherlands Cardiac Safety Services Market By End-User
10.3.3.11 Switzerland
10.3.3.11.1 Switzerland Cardiac Safety Services Market By Type
10.3.3.11.2 Switzerland Cardiac Safety Services Market By Service
10.3.3.11.3 Switzerland Cardiac Safety Services Market By End-User
10.3.3.12 Austria
10.3.3.12.1 Austria Cardiac Safety Services Market By Type
10.3.3.12.2 Austria Cardiac Safety Services Market By Service
10.3.3.12.3 Austria Cardiac Safety Services Market By End-User
10.3.3.13 Rest of Western Europe
10.3.3.13.1 Rest of Western Europe Cardiac Safety Services Market By Type
10.3.3.13.2 Rest of Western Europe Cardiac Safety Services Market By Service
10.3.3.13.3 Rest of Western Europe Cardiac Safety Services Market By End-User
10.4 Asia-Pacific
10.4.1 Trend Analysis
10.4.2 Asia-Pacific Cardiac Safety Services Market by Country
10.4.3 Asia-Pacific Cardiac Safety Services Market By Type
10.4.4 Asia-Pacific Cardiac Safety Services Market By Service
10.4.5 Asia-Pacific Cardiac Safety Services Market By End-User
10.4.6 China
10.4.6.1 China Cardiac Safety Services Market By Type
10.4.6.2 China Cardiac Safety Services Market By Service
10.4.6.3 China Cardiac Safety Services Market By End-User
10.4.7 India
10.4.7.1 India Cardiac Safety Services Market By Type
10.4.7.2 India Cardiac Safety Services Market By Service
10.4.7.3 India Cardiac Safety Services Market By End-User
10.4.8 Japan
10.4.8.1 Japan Cardiac Safety Services Market By Type
10.4.8.2 Japan Cardiac Safety Services Market By Service
10.4.8.3 Japan Cardiac Safety Services Market By End-User
10.4.9 South Korea
10.4.9.1 South Korea Cardiac Safety Services Market By Type
10.4.9.2 South Korea Cardiac Safety Services Market By Service
10.4.9.3 South Korea Cardiac Safety Services Market By End-User
10.4.10 Vietnam
10.4.10.1 Vietnam Cardiac Safety Services Market By Type
10.4.10.2 Vietnam Cardiac Safety Services Market By Service
10.4.10.3 Vietnam Cardiac Safety Services Market By End-User
10.4.11 Singapore
10.4.11.1 Singapore Cardiac Safety Services Market By Type
10.4.11.2 Singapore Cardiac Safety Services Market By Service
10.4.11.3 Singapore Cardiac Safety Services Market By End-User
10.4.12 Australia
10.4.12.1 Australia Cardiac Safety Services Market By Type
10.4.12.2 Australia Cardiac Safety Services Market By Service
10.4.12.3 Australia Cardiac Safety Services Market By End-User
10.4.13 Rest of Asia-Pacific
10.4.13.1 Rest of Asia-Pacific Cardiac Safety Services Market By Type
10.4.13.2 Rest of Asia-Pacific Cardiac Safety Services Market By Service
10.4.13.3 Rest of Asia-Pacific Cardiac Safety Services Market By End-User
10.5 Middle East & Africa
10.5.1 Trend Analysis
10.5.2 Middle East
10.5.2.1 Middle East Cardiac Safety Services Market by Country
10.5.2.2 Middle East Cardiac Safety Services Market By Type
10.5.2.3 Middle East Cardiac Safety Services Market By Service
10.5.2.4 Middle East Cardiac Safety Services Market By End-User
10.5.2.5 UAE
10.5.2.5.1 UAE Cardiac Safety Services Market By Type
10.5.2.5.2 UAE Cardiac Safety Services Market By Service
10.5.2.5.3 UAE Cardiac Safety Services Market By End-User
10.5.2.6 Egypt
10.5.2.6.1 Egypt Cardiac Safety Services Market By Type
10.5.2.6.2 Egypt Cardiac Safety Services Market By Service
10.5.2.6.3 Egypt Cardiac Safety Services Market By End-User
10.5.2.7 Saudi Arabia
10.5.2.7.1 Saudi Arabia Cardiac Safety Services Market By Type
10.5.2.7.2 Saudi Arabia Cardiac Safety Services Market By Service
10.5.2.7.3 Saudi Arabia Cardiac Safety Services Market By End-User
10.5.2.8 Qatar
10.5.2.8.1 Qatar Cardiac Safety Services Market By Type
10.5.2.8.2 Qatar Cardiac Safety Services Market By Service
10.5.2.8.3 Qatar Cardiac Safety Services Market By End-User
10.5.2.9 Rest of Middle East
10.5.2.9.1 Rest of Middle East Cardiac Safety Services Market By Type
10.5.2.9.2 Rest of Middle East Cardiac Safety Services Market By Service
10.5.2.9.3 Rest of Middle East Cardiac Safety Services Market By End-User
10.5.3 Africa
10.5.3.1 Africa Cardiac Safety Services Market by Country
10.5.3.2 Africa Cardiac Safety Services Market By Type
10.5.3.3 Africa Cardiac Safety Services Market By Service
10.5.3.4 Africa Cardiac Safety Services Market By End-User
10.5.3.5 Nigeria
10.5.3.5.1 Nigeria Cardiac Safety Services Market By Type
10.5.3.5.2 Nigeria Cardiac Safety Services Market By Service
10.5.3.5.3 Nigeria Cardiac Safety Services Market By End-User
10.5.3.6 South Africa
10.5.3.6.1 South Africa Cardiac Safety Services Market By Type
10.5.3.6.2 South Africa Cardiac Safety Services Market By Service
10.5.3.6.3 South Africa Cardiac Safety Services Market By End-User
10.5.3.7 Rest of Africa
10.5.3.7.1 Rest of Africa Cardiac Safety Services Market By Type
10.5.3.7.2 Rest of Africa Cardiac Safety Services Market By Service
10.5.3.7.3 Rest of Africa Cardiac Safety Services Market By End-User
10.6 Latin America
10.6.1 Trend Analysis
10.6.2 Latin America Cardiac Safety Services Market by country
10.6.3 Latin America Cardiac Safety Services Market By Type
10.6.4 Latin America Cardiac Safety Services Market By Service
10.6.5 Latin America Cardiac Safety Services Market By End-User
10.6.6 Brazil
10.6.6.1 Brazil Cardiac Safety Services Market By Type
10.6.6.2 Brazil Cardiac Safety Services Market By Service
10.6.6.3 Brazil Cardiac Safety Services Market By End-User
10.6.7 Argentina
10.6.7.1 Argentina Cardiac Safety Services Market By Type
10.6.7.2 Argentina Cardiac Safety Services Market By Service
10.6.7.3 Argentina Cardiac Safety Services Market By End-User
10.6.8 Colombia
10.6.8.1 Colombia Cardiac Safety Services Market By Type
10.6.8.2 Colombia Cardiac Safety Services Market By Service
10.6.8.3 Colombia Cardiac Safety Services Market By End-User
10.6.9 Rest of Latin America
10.6.9.1 Rest of Latin America Cardiac Safety Services Market By Type
10.6.9.2 Rest of Latin America Cardiac Safety Services Market By Service
10.6.9.3 Rest of Latin America Cardiac Safety Services Market By End-User
11. Company Profiles
11.1 Ncardia AG
11.1.1 Company Overview
11.1.2 Financial
11.1.3 Products/ Services Offered
11.1.4 The SNS View
11.2 Celerion
11.2.1 Company Overview
11.2.2 Financial
11.2.3 Products/ Services Offered
11.2.4 The SNS View
11.3 Certara
11.3.1 Company Overview
11.3.2 Financial
11.3.3 Products/ Services Offered
11.3.4 The SNS View
11.4 Clario
11.4.1 Company Overview
11.4.2 Financial
11.4.3 Products/ Services Offered
11.4.4 The SNS View
11.5 Richmond Pharmacology
11.5.1 Company Overview
11.5.2 Financial
11.5.3 Products/ Services Offered
11.5.4 The SNS View
11.6 Koninklijke Philips N.V.(BioTelemetry)
11.6.1 Company Overview
11.6.2 Financial
11.6.3 Products/ Services Offered
11.6.4 The SNS View
11.7 Laboratory Corporation of America Holdings
11.7.1 Company Overview
11.7.2 Financial
11.7.3 Products/ Services Offered
11.7.4 The SNS View
11.8 Medpace
11.8.1 Company Overview
11.8.2 Financial
11.8.3 Products/ Services Offered
11.8.4 The SNS View
11.9 Biotrial
11.9.1 Company Overview
11.9.2 Financial
11.9.3 Products/ Services Offered
11.9.4 The SNS View
11.10 PhysioStim
11.10.1 Company Overview
11.10.2 Financial
11.10.3 Products/ Services Offered
11.10.4 The SNS View
12. Competitive Landscape
12.1 Competitive Benchmarking
12.2 Market Share Analysis
12.3 Recent Developments
12.3.1 Industry News
12.3.2 Company News
12.3.3 Mergers & Acquisitions
13. Use Case and Best Practices
14. Conclusion
An accurate research report requires proper strategizing as well as implementation. There are multiple factors involved in the completion of good and accurate research report and selecting the best methodology to compete the research is the toughest part. Since the research reports we provide play a crucial role in any company’s decision-making process, therefore we at SNS Insider always believe that we should choose the best method which gives us results closer to reality. This allows us to reach at a stage wherein we can provide our clients best and accurate investment to output ratio.
Each report that we prepare takes a timeframe of 350-400 business hours for production. Starting from the selection of titles through a couple of in-depth brain storming session to the final QC process before uploading our titles on our website we dedicate around 350 working hours. The titles are selected based on their current market cap and the foreseen CAGR and growth.
The 5 steps process:
Step 1: Secondary Research:
Secondary Research or Desk Research is as the name suggests is a research process wherein, we collect data through the readily available information. In this process we use various paid and unpaid databases which our team has access to and gather data through the same. This includes examining of listed companies’ annual reports, Journals, SEC filling etc. Apart from this our team has access to various associations across the globe across different industries. Lastly, we have exchange relationships with various university as well as individual libraries.

Step 2: Primary Research
When we talk about primary research, it is a type of study in which the researchers collect relevant data samples directly, rather than relying on previously collected data. This type of research is focused on gaining content specific facts that can be sued to solve specific problems. Since the collected data is fresh and first hand therefore it makes the study more accurate and genuine.
We at SNS Insider have divided Primary Research into 2 parts.
Part 1 wherein we interview the KOLs of major players as well as the upcoming ones across various geographic regions. This allows us to have their view over the market scenario and acts as an important tool to come closer to the accurate market numbers. As many as 45 paid and unpaid primary interviews are taken from both the demand and supply side of the industry to make sure we land at an accurate judgement and analysis of the market.
This step involves the triangulation of data wherein our team analyses the interview transcripts, online survey responses and observation of on filed participants. The below mentioned chart should give a better understanding of the part 1 of the primary interview.

Part 2: In this part of primary research the data collected via secondary research and the part 1 of the primary research is validated with the interviews from individual consultants and subject matter experts.
Consultants are those set of people who have at least 12 years of experience and expertise within the industry whereas Subject Matter Experts are those with at least 15 years of experience behind their back within the same space. The data with the help of two main processes i.e., FGDs (Focused Group Discussions) and IDs (Individual Discussions). This gives us a 3rd party nonbiased primary view of the market scenario making it a more dependable one while collation of the data pointers.
Step 3: Data Bank Validation
Once all the information is collected via primary and secondary sources, we run that information for data validation. At our intelligence centre our research heads track a lot of information related to the market which includes the quarterly reports, the daily stock prices, and other relevant information. Our data bank server gets updated every fortnight and that is how the information which we collected using our primary and secondary information is revalidated in real time.

Step 4: QA/QC Process
After all the data collection and validation our team does a final level of quality check and quality assurance to get rid of any unwanted or undesired mistakes. This might include but not limited to getting rid of the any typos, duplication of numbers or missing of any important information. The people involved in this process include technical content writers, research heads and graphics people. Once this process is completed the title gets uploader on our platform for our clients to read it.
Step 5: Final QC/QA Process:
This is the last process and comes when the client has ordered the study. In this process a final QA/QC is done before the study is emailed to the client. Since we believe in giving our clients a good experience of our research studies, therefore, to make sure that we do not lack at our end in any way humanly possible we do a final round of quality check and then dispatch the study to the client.
By Type
Integrated
Stand-alone
By Service
ECG/Holter Measurement
Blood Pressure Measurement
Cardiovascular Imaging
Thorough QT Studies
Others
By End User
Pharmaceutical and Biopharmaceutical Companies
Contract Research Organizations
Others
Request for Segment Customization as per your Business Requirement: Segment Customization Request
Regional Coverage:
North America
US
Canada
Mexico
Europe
Eastern Europe
Poland
Romania
Hungary
Turkey
Rest of Eastern Europe
Western Europe
Germany
France
UK
Italy
Spain
Netherlands
Switzerland
Austria
Rest of Western Europe
Asia Pacific
China
India
Japan
South Korea
Vietnam
Singapore
Australia
Rest of Asia Pacific
Middle East & Africa
Middle East
UAE
Egypt
Saudi Arabia
Qatar
Rest of Middle East
Africa
Nigeria
South Africa
Rest of Africa
Latin America
Brazil
Argentina
Colombia
Rest of Latin America
Request for Country Level Research Report: Country Level Customization Request
Available Customization
With the given market data, SNS Insider offers customization as per the company’s specific needs. The following customization options are available for the report:
Product Analysis
Criss-Cross segment analysis (e.g. Product X Application)
Product Matrix which gives a detailed comparison of product portfolio of each company
Geographic Analysis
Additional countries in any of the regions
Company Information
Detailed analysis and profiling of additional market players (Up to five)
The Dental Tourism Market was valued at USD 10.91 billion in 2023 and is expected to reach USD 65.4 billion by 2032, growing at a CAGR of 22.03% over the forecast period 2024-2032.
Sports Medicine Market size was valued at USD 5.69 billion in 2023, and is expected to grow to USD 10.05 billion by 2032 at a CAGR of 6.54% from 2024-2032.
The Infusion Pump Software Market size was USD 862.12 Million in 2023 and is expected to reach USD 1655.17 Million by 2032 and grow at a CAGR of 7.52% over the forecast period of 2024-2032.
The 3D Printed Brain Model Market was valued at USD 50.00 million in 2023 and is expected to reach USD 235.42 million by 2032, growing at a CAGR of 18.39% from 2024-2032.
The HPV Testing And Pap Test Market Size was valued at USD 4.95 Billion in 2023 and is expected to reach USD 14.31 Billion by 2032 and grow at a CAGR of 12.54% over the forecast period 2024-2032.
The Clinical Trial Management System Market Size was valued at USD 1.80 Billion in 2023 and is expected to reach USD 5.93 Billion by 2032, growing at a CAGR of 14.18% over the forecast period 2024-2032.
Hi! Click one of our member below to chat on Phone