Get More Information on Cancer Biologics Market - Request Sample Report
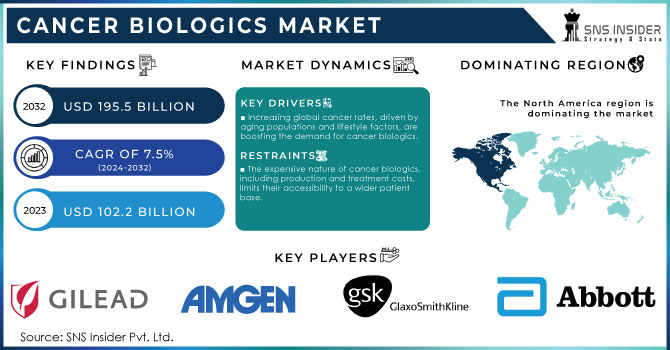
The Cancer Biologics Market Size was valued at USD 102.2 Billion in 2023 and is expected to reach USD 195.5 Bn by 2032, with a growing CAGR of 7.5% Over the Forecast Period of 2024-2032.
The global cancer biologics market is witnessing high growth due to the increasing prevalence of different types of cancers around the world and the rising number of government initiatives for improving healthcare services by using more effective treatment. In 2023, according to the latest statistics of the World Health Organization (WHO), there were around over 19.3 million new cancer cases and also more than 10 million deaths caused by cancers. Governments globally are stepping up efforts to address this growing health crisis. The U.S. National Cancer Institute (NCI) has estimated that government funding for cancer research reached $7.6 billion in 2023, a gain of 8% over the previous year.
Monoclonal antibodies, vaccines, and gene therapies that constitute biological treatments are more effective with fewer side effects when compared to traditional chemotherapy making them revolutionary in oncology. The governments in both Europe and the Asia-Pacific regions have launched substantial campaigns to streamline clinical trials as well as regulatory approvals for biologics. For example, the European Medicines Agency (EMA) expedited approvals of a number of cancer biologics in 2023, and China's National Medical Products Administration (NMPA) saw a record number of biologics approved in 2023 as part of its expanded healthcare reforms. The supportive regulations and increasing awareness about personalized medicine are anticipated to keep the demand for cancer biologics. As governments continue to focus on healthcare infrastructure and increase their spending on advanced therapies, the market is poised for significant expansion in the coming years.
Drivers
Increasing global cancer rates, driven by aging populations and lifestyle factors, are boosting the demand for cancer biologics.
Continuous innovations in biotechnology and targeted therapies, such as monoclonal antibodies and CAR-T cell therapies, are expanding treatment options.
Growing support from governments and non-governmental organizations (NGOs) for cancer research, drug development, and awareness programs is fuelling market growth.
Fast-track approvals and orphan drug designations for novel biologics by regulatory bodies like the FDA and EMA are accelerating product launches.
Increasing focus on personalized and precision medicine is encouraging the development of biologics tailored to individual cancer profiles.
The global cancer biologics market is driven by the rising global prevalence of cancer, especially because the world population is aging and many environmental factors are making it easier for people to get diagnosed with some form of this disease. Cancer, which the World Health Organization (WHO) still lists as one of its leading causes for death since most cancer types are detected late stage. With the growing burden of cancer, demand for novel biological cancer treatments is on the rise due to their precision and ability to target cancer cells with minimal damage to surrounding healthy tissue.
Global governments are already addressing the crisis through escalated financial funding and support for cancer research and biological drug development. The U.S. National Cancer Institute (NCI), part of the National Institutes of Health (NIH) is anticipated to budget $6.9 billion for cancer research in fiscal year 2023. This funding aims to foster innovation in therapies, including biologics such as monoclonal antibodies and CAR-T cell therapies. They are also increasing speed regulations for government approval. In 2023, the U.S. FDA granted priority review status to several cancer biologics, expediting their approval and market entry. At the same time, orphan drug designations for biologics against rare cancers are being granted by the European Medicines Agency (EMEA), which means these drugs will be considered to receive individual treatment. Such initiatives account for a major share in the innovation and application of biologics, giving promising signs to global cancer therapy.
Restraints
The expensive nature of cancer biologics, including production and treatment costs, limits their accessibility to a wider patient base.
The production of biologics is complex and requires advanced facilities, which can lead to manufacturing delays and supply chain issues.
The rigorous and time-consuming regulatory approval process for biologics can slow down market entry and increase development costs.
The expiration of key biologic patents opens up the market to biosimilars, leading to increased competition and potential revenue decline for branded biologics.
The high cost associated with treating patients using cancer biologics is one of the critical challenges faced by players operating in this market. Monoclonal antibodies, CAR-T cell therapies, and immunotherapies are among the different types of cancer biologics that are notoriously difficult to develop and manufacture. Being produced through a complex biological process, their manufacturing necessitates strict infrastructure requirements: highly qualified workers and aggressive quality standards. This leads to high production costs, which are passed on to patients, making these treatments expensive. For many patients, the cost can be prohibitive, especially in low- and middle-income regions where healthcare coverage is limited. In even well-developed countries this increases cost and reduces access. More importantly, the complete cost of these advanced therapies might not be covered by insurance companies which could further make it difficult for patients who really need those treatments. This places financial pressure on the marketplace and limits equal access to innovative cancer treatments.
By Application
The blood cancer segment dominated the market share in 2023 and accounted for 25% of the market share due to the increasing number of cases associated with blood cancers, such as leukemia and lymphoma in addition to myelomas. In 2023, an estimated 1.3 million people in the United States are expected to be living with or in remission from blood cancer including leukemia and lymphoma depending on estimates of disease prevalence made available by The Leukemia & Lymphoma Society (LLS). The US government is driving this with significant investment earmarked for blood cancer research by the National Cancer Institute alone allocating over $500 million for blood cancer research in 2023. Owing to the significant advantages in treatment outcomes that these novel agents provide, inherently there is more demand which has propelled a rapid market growth as well. Breast cancer accounts significant share of the cancer biologics market, because of the high proportion of breast cancer cases. Around the world, 2.3 million new cases of breast cancer have been diagnosed (WHO). In that context, governmental actions to increase coverage and improve early diagnosis and treatment are very important. Examples of this would be how in the US, organizations such as The National Institutes of Health (NIH) and others have upped funding for breast cancer screening programs, along with labeling very expensive biological treatments a top priority. This trend will likely be supported by the use of newer biologics namely, monoclonal antibodies (mAbs), immune checkpoint inhibitors, and antibody-drug conjugates that have been specifically developed for breast cancer indication.
The lung cancer segment is witnessing the highest compound annual growth rate (CAGR), due to the rising incidence of this cancer globally and the increasing adoption of biologics in its treatment. According to the World Health Organization (WHO), Lung Cancer is the main cause of death related to cancers with over 2.2 million new cases diagnosed annually. Government health agencies are actively promoting the use of biologics to improve treatment outcomes, especially for advanced-stage lung cancer patients. Lung cancer biologics, including immune checkpoint inhibitors and targeted therapies, have shown significant potential in improving survival rates, which is driving the rapid growth of this segment.
By End-User
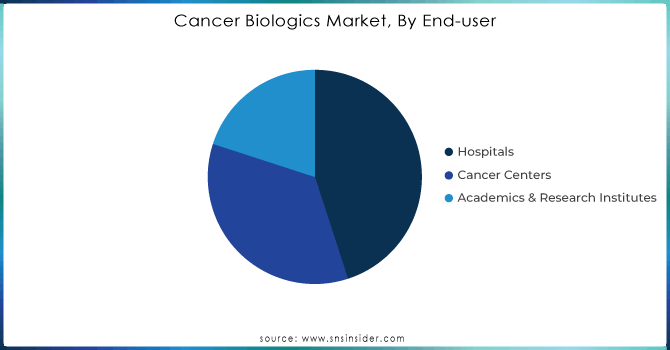
Hospitals held the largest market share more than 45% of the cancer biologics market. This dominance is attributed to the extensive infrastructure available in hospitals for administering complex biological therapies, which often require specialized facilities and expertise. The capacity of hospitals to deliver state-of-the-art cancer treatments has been enhanced by government policy and funding. On the one hand, the U.S. government’s investment in cancer hospitals has been significant, with an estimated $4 billion in funding allocated through the National Cancer Institute’s Cancer Centers Program in 2023. This funding ensures that hospitals remain at the forefront of cancer treatment, providing the latest biological therapies to patients. Hospitals also benefit from government grants aimed at expanding oncology departments and purchasing advanced biologic therapies. The growing acceptance of biologics in cancer care protocols could keep the segment at the top position, underpinned by sustained government funding and healthcare infrastructure investments that favor hospitals.
The cancer centers segment is expected to grow at the highest CAGR during 2024-2032, driven by a growing preference towards a focus on specialized health concerns and growth in a number of standalone cancer treatment centers across the globe. Demand is surging at cancer centers dedicated to providing the latest biologic therapies as part of an integrated approach to caring for increasingly sophisticated patients. By 2023, there were more than 70 designated cancer centers in the United States categorized as the National Cancer Institute (NCI) and has helped some new facilities to gain government funding for boosting their biologics treatment abilities.
Need any customization research on Cancer Biologics Market - Enquiry Now
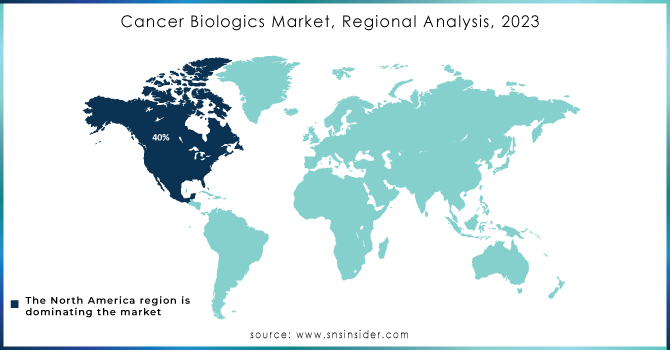
North America dominated the cancer biologics market in 2023 and accounted for more than 40% of the market share in 2023. The dominance of the region is attributable to the high prevalence of cancer, well-established healthcare infrastructure, and heavy government funding for research in oncology. In particular, the United States has experienced major governmental spending in biologics with the National Cancer Moonshot initiative setting ambitious goals to reduce cancer deaths through early detection and innovative therapies, including biologics. Furthermore, the U.S. Food and Drug Administration (FDA) has created shorter regulatory pathways for biological drugs which lead to additional market growth throughout the forecast period. We also know that cancer biologics and their spending have increased in Canada, with the government offering supplementary payments to fund its programs on a large scale. Together, favorable government policies along with a high degree of innovation and increasing incidence of cancer cases are keeping North America in the dominating domain across the global market for the sale of biologics.
The major players in the market are Gilead Sciences, Inc., F. Hoffmann-La Roche Ltd., Amgen, Inc., GSK plc., Eli Lilly and Company, Bristol-Myers Squibb Company, Pfizer, Inc., Abbott, AstraZeneca, Johnson & Johnson Services, Inc., and others in final Report.
July 2023 - U.S. Food and Drug Administration (FDA): The FDA fast-tracked the approval of a new monoclonal antibody treatment for blood cancer, developed under the Orphan Drug Designation program. This biologic therapy has shown a 25% improvement in remission rates in clinical trials and is expected to be available for patient use by early 2024.
May 2023 – European Medicines Agency (EMA) approved two new products used to treat lung and colorectal cancers. The European Commission report said the therapies could increase survival rates by 15-20% for people with advanced-stage cancers.
January 2024: European Commission approves for Roche’s Tecentriq SC, marking the EU's first PD-L1 cancer immunotherapy available by subcutaneous injection in Europe across multiple cancer types
March 2024: Novartis Presents New Data Demonstrating Sustained and Improved Motor Milestones in Older, Heavier Children with Spinal Muscular Atrophy (SMA) Treated Early With Zolgensma
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | USD 102.2 Billion |
| Market Size by 2032 | USD 195.5 Billion |
| CAGR | CAGR of 7.5% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Drug Class (Monoclonal Antibodies (mAb), Recombinants Proteins, Cancer Growth Inhibitors, Vaccines, CAR-T Cells, Angiogenesis Inhibitors, Interleukins (IL), Others) • By Application (Blood Cancer, Lung Cancer, Breast Cancer, Colorectal Cancer, Prostate Cancer, Gastric Cancer, Ovarian Cancer, Others) • By End-user (Hospitals, Cancer Centers, Academics & Research Institutes) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | Canon Medical Systems Corporation, Siemens Healthcare GmbH, Ziehm Imaging GmbH, Medtronic plc, GE HealthCare, IMRIS, Brainlab AG, Koninklijke Philips N.V., NeuroLogica Corp, Shimadzu Corporation (Medical Systems). |
| Key Drivers | • Increasing global cancer rates, driven by aging populations and lifestyle factors, are boosting the demand for cancer biologics. • Continuous innovations in biotechnology and targeted therapies, such as monoclonal antibodies and CAR-T cell therapies, are expanding treatment options. |
| RESTRAINTS | • The expensive nature of cancer biologics, including production and treatment costs, limits their accessibility to a wider patient base. • The production of biologics is complex and requires advanced facilities, which can lead to manufacturing delays and supply chain issues. |
Ans. The projected market size for the Cancer Biologics Market is USD 195.5 billion by 2032.
Ans. The CAGR of the Cancer Biologics Market is 7.5% During the forecast period of 2024-2032.
Ans: The Hospitals end user segment dominated the Cancer Biologics Market.
Ans: Yes, you can customize the report as per your requirements.
Ans: The North American region dominated the Cancer Biologics Market in 2023.
Table of Contents
1. Introduction
1.1 Market Definition
1.2 Scope (Inclusion and Exclusions)
1.3 Research Assumptions
2. Executive Summary
2.1 Market Overview
2.2 Regional Synopsis
2.3 Competitive Summary
3. Research Methodology
3.1 Top-Down Approach
3.2 Bottom-up Approach
3.3. Data Validation
3.4 Primary Interviews
4. Market Dynamics Impact Analysis
4.1 Market Driving Factors Analysis
4.1.1 Drivers
4.1.2 Restraints
4.1.3 Opportunities
4.1.4 Challenges
4.2 PESTLE Analysis
4.3 Porter’s Five Forces Model
5. Statistical Insights and Trends Reporting
5.1 Incidence and Prevalence (2023)
5.2 Prescription Trends, (2023), by Region
5.3 Drug Volume: Production and usage volumes of pharmaceuticals.
5.4 Healthcare Spending: Expenditure data by government, insurers, and out-of-pocket by patients.
6. Competitive Landscape
6.1 List of Major Companies, By Region
6.2 Market Share Analysis, By Region
6.3 Product Benchmarking
6.3.1 Product specifications and features
6.3.2 Pricing
6.4 Strategic Initiatives
6.4.1 Marketing and promotional activities
6.4.2 Distribution and supply chain strategies
6.4.3 Expansion plans and new product launches
6.4.4 Strategic partnerships and collaborations
6.5 Technological Advancements
6.6 Market Positioning and Branding
7. Cancer Biologics Market Segmentation, By Drug Class
7.1 Chapter Overview
7.2 Monoclonal Antibodies (mAb)
7.2.1 Monoclonal Antibodies (mAb) Market Trends Analysis (2020-2032)
7.2.2 Monoclonal Antibodies (mAb) Market Size Estimates and Forecasts to 2032 (USD Billion)
7.2.3 Naked mAb
7.2.3.1 Naked mAb Market Trends Analysis (2020-2032)
7.2.3.2 Naked mAb Market Size Estimates and Forecasts to 2032 (USD Billion)
7.2.4 Conjugated mAb
7.2.4.1 Conjugated mAb Market Trends Analysis (2020-2032)
7.2.4.2 Conjugated mAb Market Size Estimates and Forecasts to 2032 (USD Billion)
7.2.5 Bispecific mAb
7.2.5.1 Bispecific mAb Market Trends Analysis (2020-2032)
7.2.5.2 Bispecific mAb Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3 Recombinants Proteins
7.3.1 Recombinants Proteins Market Trends Analysis (2020-2032)
7.3.2 Recombinants Proteins Market Size Estimates and Forecasts to 2032 (USD Billion)
7.4 Cancer Growth Inhibitors
7.4.1 Cancer Growth Inhibitors Market Trends Analysis (2020-2032)
7.4.2 Cancer Growth Inhibitors Market Size Estimates and Forecasts to 2032 (USD Billion)
7.4.3 Tyrosine Kinase Inhibitors
7.4.3.1 Tyrosine Kinase Inhibitors Market Trends Analysis (2020-2032)
7.4.3.2 Tyrosine Kinase Inhibitors Market Size Estimates and Forecasts to 2032 (USD Billion)
7.4.4 mTOR Inhibitors
7.4.4.1 mTOR Inhibitors Market Trends Analysis (2020-2032)
7.4.4.2 mTOR Inhibitors Market Size Estimates and Forecasts to 2032 (USD Billion)
7.4.5 Others
7.4.5.1 Others Market Trends Analysis (2020-2032)
7.4.5.2 Others Market Size Estimates and Forecasts to 2032 (USD Billion)
7.5 Vaccines
7.5.1 Vaccines Market Trends Analysis (2020-2032)
7.5.2 Vaccines Market Size Estimates and Forecasts to 2032 (USD Billion)
7.5.3 Preventive Vaccines
7.5.3.1 Preventive Vaccines Market Trends Analysis (2020-2032)
7.5.3.2 Preventive Vaccines Market Size Estimates and Forecasts to 2032 (USD Billion)
7.5.4 Therapeutic Vaccines
7.5.4.1 Therapeutic Vaccines Market Trends Analysis (2020-2032)
7.5.4.2 Therapeutic Vaccines Market Size Estimates and Forecasts to 2032 (USD Billion)
7.6 CAR-T Cells
7.6.1 CAR-T Cells Market Trends Analysis (2020-2032)
7.6.2 CAR-T Cells Market Size Estimates and Forecasts to 2032 (USD Billion)
7.7 Angiogenesis Inhibitors
7.7.1 Angiogenesis Inhibitors Market Trends Analysis (2020-2032)
7.7.2 Angiogenesis Inhibitors Market Size Estimates and Forecasts to 2032 (USD Billion)
7.8 Interleukins (IL)
7.8.1 Interleukins (IL) Market Trends Analysis (2020-2032)
7.8.2 Interleukins (IL) Market Size Estimates and Forecasts to 2032 (USD Billion)
7.9 Others
7.9.1 Others Market Trends Analysis (2020-2032)
7.9.2 Others Market Size Estimates and Forecasts to 2032 (USD Billion)
8. Cancer Biologics Market Segmentation, By Application
8.1 Chapter Overview
8.2 Blood Cancer
8.2.1 Blood Cancer Market Trends Analysis (2020-2032)
8.2.2 Blood Cancer Market Size Estimates and Forecasts to 2032 (USD Billion)
8.3 Lung Cancer
8.3.1 Lung Cancer Market Trends Analysis (2020-2032)
8.3.2 Lung Cancer Market Size Estimates and Forecasts to 2032 (USD Billion)
8.4 Breast Cancer
8.4.1 Breast Cancer Market Trends Analysis (2020-2032)
8.4.2 Breast Cancer Market Size Estimates and Forecasts to 2032 (USD Billion)
8.5 Colorectal Cancer
8.5.1 Colorectal Cancer Market Trends Analysis (2020-2032)
8.5.2 Colorectal Cancer Market Size Estimates and Forecasts to 2032 (USD Billion)
8.6 Prostate Cancer
8.6.1 Prostate Cancer Market Trends Analysis (2020-2032)
8.6.2 Prostate Cancer Market Size Estimates and Forecasts to 2032 (USD Billion)
8.7 Gastric Cancer
8.7.1 Gastric Cancer Market Trends Analysis (2020-2032)
8.7.2 Gastric Cancer Market Size Estimates and Forecasts to 2032 (USD Billion)
8.8 Ovarian Cancer
8.8.1 Ovarian Cancer Market Trends Analysis (2020-2032)
8.8.2 Ovarian Cancer Market Size Estimates and Forecasts to 2032 (USD Billion)
8.9 Others
8.9.1 Others Market Trends Analysis (2020-2032)
8.9.2 Others Market Size Estimates and Forecasts to 2032 (USD Billion)
9. Cancer Biologics Market Segmentation, By End-user
9.1 Chapter Overview
9.2 Hospitals
9.2.1 Hospitals Market Trends Analysis (2020-2032)
9.2.2 Hospitals Market Size Estimates and Forecasts to 2032 (USD Billion)
9.3 Cancer Centers
9.3.1 Cancer Centers Market Trends Analysis (2020-2032)
9.3.2 Cancer Centers Market Size Estimates and Forecasts to 2032 (USD Billion)
9.4 Academics & Research Institutes
9.4.1 Academics & Research Institutes Market Trends Analysis (2020-2032)
9.4.2 Academics & Research Institutes Market Size Estimates and Forecasts to 2032 (USD Billion)
10. Regional Analysis
10.1 Chapter Overview
10.2 North America
10.2.1 Trends Analysis
10.2.2 North America Cancer Biologics Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.2.3 North America Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.2.4 North America Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.2.5 North America Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.2.6 USA
10.2.6.1 USA Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.2.6.2 USA Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.2.6.3 USA Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.2.7 Canada
10.2.7.1 Canada Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.2.7.2 Canada Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.2.7.3 Canada Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.2.8 Mexico
10.2.8.1 Mexico Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.2.8.2 Mexico Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.2.8.3 Mexico Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.3 Europe
10.3.1 Eastern Europe
10.3.1.1 Trends Analysis
10.3.1.2 Eastern Europe Cancer Biologics Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.3.1.3 Eastern Europe Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.3.1.4 Eastern Europe Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.3.1.5 Eastern Europe Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.3.1.6 Poland
10.3.1.6.1 Poland Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.3.1.6.2 Poland Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.3.1.6.3 Poland Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.3.1.7 Romania
10.3.1.7.1 Romania Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.3.1.7.2 Romania Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.3.1.7.3 Romania Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.3.1.8 Hungary
10.3.1.8.1 Hungary Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.3.1.8.2 Hungary Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.3.1.8.3 Hungary Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.3.1.9 Turkey
10.3.1.9.1 Turkey Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.3.1.9.2 Turkey Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.3.1.9.3 Turkey Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.3.1.10 Rest of Eastern Europe
10.3.1.10.1 Rest of Eastern Europe Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.3.1.10.2 Rest of Eastern Europe Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.3.1.10.3 Rest of Eastern Europe Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.3.2 Western Europe
10.3.2.1 Trends Analysis
10.3.2.2 Western Europe Cancer Biologics Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.3.2.3 Western Europe Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.3.2.4 Western Europe Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.3.2.5 Western Europe Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.3.2.6 Germany
10.3.2.6.1 Germany Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.3.2.6.2 Germany Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.3.2.6.3 Germany Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.3.2.7 France
10.3.2.7.1 France Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.3.2.7.2 France Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.3.2.7.3 France Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.3.2.8 UK
10.3.2.8.1 UK Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.3.2.8.2 UK Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.3.2.8.3 UK Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.3.2.9 Italy
10.3.2.9.1 Italy Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.3.2.9.2 Italy Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.3.2.9.3 Italy Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.3.2.10 Spain
10.3.2.10.1 Spain Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.3.2.10.2 Spain Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.3.2.10.3 Spain Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.3.2.11 Netherlands
10.3.2.11.1 Netherlands Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.3.2.11.2 Netherlands Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.3.2.11.3 Netherlands Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.3.2.12 Switzerland
10.3.2.12.1 Switzerland Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.3.2.12.2 Switzerland Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.3.2.12.3 Switzerland Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.3.2.13 Austria
10.3.2.13.1 Austria Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.3.2.13.2 Austria Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.3.2.13.3 Austria Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.3.2.14 Rest of Western Europe
10.3.2.14.1 Rest of Western Europe Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.3.2.14.2 Rest of Western Europe Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.3.2.14.3 Rest of Western Europe Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.4 Asia Pacific
10.4.1 Trends Analysis
10.4.2 Asia Pacific Cancer Biologics Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.4.3 Asia Pacific Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.4.4 Asia Pacific Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.4.5 Asia Pacific Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.4.6 China
10.4.6.1 China Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.4.6.2 China Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.4.6.3 China Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.4.7 India
10.4.7.1 India Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.4.7.2 India Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.4.7.3 India Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.4.8 Japan
10.4.8.1 Japan Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.4.8.2 Japan Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.4.8.3 Japan Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.4.9 South Korea
10.4.9.1 South Korea Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.4.9.2 South Korea Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.4.9.3 South Korea Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.4.10 Vietnam
10.4.10.1 Vietnam Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.4.10.2 Vietnam Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.4.10.3 Vietnam Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.4.11 Singapore
10.4.11.1 Singapore Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.4.11.2 Singapore Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.4.11.3 Singapore Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.4.12 Australia
10.4.12.1 Australia Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.4.12.2 Australia Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.4.12.3 Australia Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.4.13 Rest of Asia Pacific
10.4.13.1 Rest of Asia Pacific Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.4.13.2 Rest of Asia Pacific Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.4.13.3 Rest of Asia Pacific Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.5 Middle East and Africa
10.5.1 Middle East
10.5.1.1 Trends Analysis
10.5.1.2 Middle East Cancer Biologics Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.5.1.3 Middle East Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.5.1.4 Middle East Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.5.1.5 Middle East Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.5.1.6 UAE
10.5.1.6.1 UAE Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.5.1.6.2 UAE Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.5.1.6.3 UAE Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.5.1.7 Egypt
10.5.1.7.1 Egypt Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.5.1.7.2 Egypt Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.5.1.7.3 Egypt Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.5.1.8 Saudi Arabia
10.5.1.8.1 Saudi Arabia Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.5.1.8.2 Saudi Arabia Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.5.1.8.3 Saudi Arabia Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.5.1.9 Qatar
10.5.1.9.1 Qatar Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.5.1.9.2 Qatar Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.5.1.9.3 Qatar Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.5.1.10 Rest of Middle East
10.5.1.10.1 Rest of Middle East Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.5.1.10.2 Rest of Middle East Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.5.1.10.3 Rest of Middle East Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.5.2 Africa
10.5.2.1 Trends Analysis
10.5.2.2 Africa Cancer Biologics Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.5.2.3 Africa Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.5.2.4 Africa Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.5.2.5 Africa Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.5.2.6 South Africa
10.5.2.6.1 South Africa Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.5.2.6.2 South Africa Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.5.2.6.3 South Africa Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.5.2.7 Nigeria
10.5.2.7.1 Nigeria Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.5.2.7.2 Nigeria Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.5.2.7.3 Nigeria Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.5.2.8 Rest of Africa
10.5.2.8.1 Rest of Africa Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.5.2.8.2 Rest of Africa Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.5.2.8.3 Rest of Africa Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.6 Latin America
10.6.1 Trends Analysis
10.6.2 Latin America Cancer Biologics Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.6.3 Latin America Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.6.4 Latin America Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.6.5 Latin America Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.6.6 Brazil
10.6.6.1 Brazil Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.6.6.2 Brazil Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.6.6.3 Brazil Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.6.7 Argentina
10.6.7.1 Argentina Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.6.7.2 Argentina Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.6.7.3 Argentina Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.6.8 Colombia
10.6.8.1 Colombia Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.6.8.2 Colombia Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.6.8.3 Colombia Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
10.6.9 Rest of Latin America
10.6.9.1 Rest of Latin America Cancer Biologics Market Estimates and Forecasts, By Drug Class (2020-2032) (USD Billion)
10.6.9.2 Rest of Latin America Cancer Biologics Market Estimates and Forecasts, By Application (2020-2032) (USD Billion)
10.6.9.3 Rest of Latin America Cancer Biologics Market Estimates and Forecasts, By End-user (2020-2032) (USD Billion)
11. Company Profiles
11.1 Gilead Sciences, Inc.
11.1.1 Company Overview
11.1.2 Financial
11.1.3 Products/ Services Offered
11.1.4 SWOT Analysis
11.2 F. Hoffmann-La Roche Ltd.
11.2.1 Company Overview
11.2.2 Financial
11.2.3 Products/ Services Offered
11.2.4 SWOT Analysis
11.3 Amgen, Inc.
11.3.1 Company Overview
11.3.2 Financial
11.3.3 Products/ Services Offered
11.3.4 SWOT Analysis
11.4 GSK plc.
11.4.1 Company Overview
11.4.2 Financial
11.4.3 Products/ Services Offered
11.4.4 SWOT Analysis
11.5 Eli Lilly and Company
11.5.1 Company Overview
11.5.2 Financial
11.5.3 Products/ Services Offered
11.5.4 SWOT Analysis
11.6 Bristol-Myers Squibb Company
11.6.1 Company Overview
11.6.2 Financial
11.6.3 Products/ Services Offered
11.6.4 SWOT Analysis
11.7 Pfizer, Inc.
11.7.1 Company Overview
11.7.2 Financial
11.7.3 Products/ Services Offered
11.7.4 SWOT Analysis
11.8 Abbott
11.8.1 Company Overview
11.8.2 Financial
11.8.3 Products/ Services Offered
11.8.4 SWOT Analysis
11.9 AstraZeneca
11.9.1 Company Overview
11.9.2 Financial
11.9.3 Products/ Services Offered
11.9.4 SWOT Analysis
11.10 Johnson & Johnson Services, Inc.
11.10.1 Company Overview
11.10.2 Financial
11.10.3 Products/ Services Offered
11.10.4 SWOT Analysis
12. Use Cases and Best Practices
13. Conclusion
An accurate research report requires proper strategizing as well as implementation. There are multiple factors involved in the completion of good and accurate research report and selecting the best methodology to compete the research is the toughest part. Since the research reports we provide play a crucial role in any company’s decision-making process, therefore we at SNS Insider always believe that we should choose the best method which gives us results closer to reality. This allows us to reach at a stage wherein we can provide our clients best and accurate investment to output ratio.
Each report that we prepare takes a timeframe of 350-400 business hours for production. Starting from the selection of titles through a couple of in-depth brain storming session to the final QC process before uploading our titles on our website we dedicate around 350 working hours. The titles are selected based on their current market cap and the foreseen CAGR and growth.
The 5 steps process:
Step 1: Secondary Research:
Secondary Research or Desk Research is as the name suggests is a research process wherein, we collect data through the readily available information. In this process we use various paid and unpaid databases which our team has access to and gather data through the same. This includes examining of listed companies’ annual reports, Journals, SEC filling etc. Apart from this our team has access to various associations across the globe across different industries. Lastly, we have exchange relationships with various university as well as individual libraries.

Step 2: Primary Research
When we talk about primary research, it is a type of study in which the researchers collect relevant data samples directly, rather than relying on previously collected data. This type of research is focused on gaining content specific facts that can be sued to solve specific problems. Since the collected data is fresh and first hand therefore it makes the study more accurate and genuine.
We at SNS Insider have divided Primary Research into 2 parts.
Part 1 wherein we interview the KOLs of major players as well as the upcoming ones across various geographic regions. This allows us to have their view over the market scenario and acts as an important tool to come closer to the accurate market numbers. As many as 45 paid and unpaid primary interviews are taken from both the demand and supply side of the industry to make sure we land at an accurate judgement and analysis of the market.
This step involves the triangulation of data wherein our team analyses the interview transcripts, online survey responses and observation of on filed participants. The below mentioned chart should give a better understanding of the part 1 of the primary interview.

Part 2: In this part of primary research the data collected via secondary research and the part 1 of the primary research is validated with the interviews from individual consultants and subject matter experts.
Consultants are those set of people who have at least 12 years of experience and expertise within the industry whereas Subject Matter Experts are those with at least 15 years of experience behind their back within the same space. The data with the help of two main processes i.e., FGDs (Focused Group Discussions) and IDs (Individual Discussions). This gives us a 3rd party nonbiased primary view of the market scenario making it a more dependable one while collation of the data pointers.
Step 3: Data Bank Validation
Once all the information is collected via primary and secondary sources, we run that information for data validation. At our intelligence centre our research heads track a lot of information related to the market which includes the quarterly reports, the daily stock prices, and other relevant information. Our data bank server gets updated every fortnight and that is how the information which we collected using our primary and secondary information is revalidated in real time.

Step 4: QA/QC Process
After all the data collection and validation our team does a final level of quality check and quality assurance to get rid of any unwanted or undesired mistakes. This might include but not limited to getting rid of the any typos, duplication of numbers or missing of any important information. The people involved in this process include technical content writers, research heads and graphics people. Once this process is completed the title gets uploader on our platform for our clients to read it.
Step 5: Final QC/QA Process:
This is the last process and comes when the client has ordered the study. In this process a final QA/QC is done before the study is emailed to the client. Since we believe in giving our clients a good experience of our research studies, therefore, to make sure that we do not lack at our end in any way humanly possible we do a final round of quality check and then dispatch the study to the client.
Key Segments:
By Drug Class
Monoclonal Antibodies (mAb)- major share
Naked mAb
Conjugated mAb
Bispecific mAb
Recombinants Proteins
Cancer Growth Inhibitors
Tyrosine Kinase Inhibitors
mTOR Inhibitors
Others (proteasome inhibitors)
Vaccines
Preventive Vaccines
Therapeutic Vaccines
CAR-T Cells
Angiogenesis Inhibitors
Interleukins (IL)
Others (interferons (IFN), gene therapy, etc.)
By Application
Blood Cancer
Lung Cancer
Breast Cancer
Colorectal Cancer
Prostate Cancer
Gastric Cancer
Ovarian Cancer
Others
By End-user
Hospitals
Cancer Centers
Academics & Research Institutes
Request for Segment Customization as per your Business Requirement: Segment Customization Request
REGIONAL COVERAGE:
North America
US
Canada
Mexico
Europe
Eastern Europe
Poland
Romania
Hungary
Turkey
Rest of Eastern Europe
Western Europe
Germany
France
UK
Italy
Spain
Netherlands
Switzerland
Austria
Rest of Western Europe
Asia Pacific
China
India
Japan
South Korea
Vietnam
Singapore
Australia
Rest of Asia Pacific
Middle East & Africa
Middle East
UAE
Egypt
Saudi Arabia
Qatar
Rest of the Middle East
Africa
Nigeria
South Africa
Rest of Africa
Latin America
Brazil
Argentina
Colombia
Request for Country Level Research Report: Country Level Customization Request
Available Customization
With the given market data, SNS Insider offers customization as per the company’s specific needs. The following customization options are available for the report:
Product Analysis
Criss-Cross segment analysis (e.g. Product X Application)
Product Matrix which gives a detailed comparison of product portfolio of each company
Geographic Analysis
Additional countries in any of the regions
Company Information
Detailed analysis and profiling of additional market players (Up to five)
The Spinal Imaging Market was valued at USD 2.04 billion in 2023 and is expected to reach USD 3.23 billion by 2032, growing at a CAGR of 5.24% over the forecast period of 2024-2032.
The Bioactive Wound Care Market was valued at USD 11.06 billion in 2023 and is expected to reach USD 21.18 billion by 2032, growing at a CAGR of 7.52% from 2024-2032.
The Companion Diagnostics Market Size, valued at USD 7.66 billion in 2023, is expected to reach USD 21.15 billion by 2032, with a CAGR of 11.9%.
The Blood Processing Devices and Consumables Market was valued at USD 48.3 billion in 2023 and is expected to reach USD 89.41 billion by 2032, growing at a CAGR of 7.11% over the forecast period of 2024-2032.
The Cardiology Information System (CIS) Market Size was valued at USD 1.09 billion in 2023 and is expected to reach USD 2.24 billion by 2031 and grow at a CAGR of 9.4% over the forecast period 2024-2031.
Sterilization Equipment Market Size was valued at USD 7.0 Billion in 2023 and is expected to reach USD 18.44 Billion by 2032, growing at a CAGR of 11.38% over the forecast period 2024-2032.
Hi! Click one of our member below to chat on Phone