To get more information on Bleeding Disorders Treatment Market - Request Free Sample Report
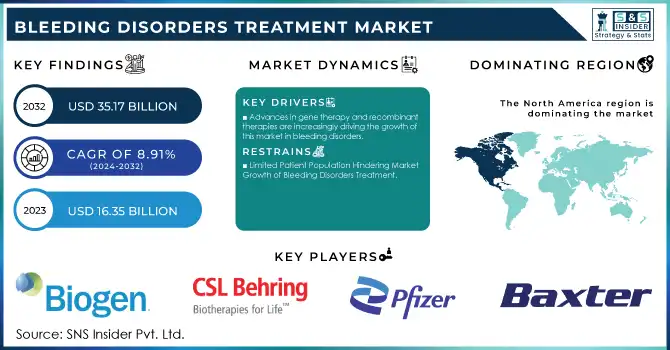
The Bleeding Disorders Treatment Market Size was valued at USD 16.35 billion in 2023 and is projected to reach USD 35.17 billion by 2032, growing at a CAGR of 8.91% from 2024-2032.
The Bleeding Disorders Treatment Market is growing enormously because of the increasing incidence of bleeding disorders such as hemophilia, Von Willebrand disease (vWD), and other rare clotting disorders. Hemophilia, primarily Hemophilia A and B, is the most common, with an estimated incidence of about 1 in 5,000 male births worldwide. This has driven the demand for advanced therapies to improve the quality of life of patients and decrease the risks associated with bleeding events.
In the past few years, gene therapy and recombinant proteins have changed the landscape of treatment. For example, CSL announced the three-year results from the HOPE-B study for etranacogene dezaparvovec-drlb, a gene therapy for Hemophilia B that has confirmed long-term safety and efficacy post one-time infusion in December 2023. Similarly, Takeda's ADAMTS13, recombinant-krhn was approved by the U.S. FDA in November 2023 approved it as the treatment for congenital thrombotic thrombocytopenic purpura or cTTP. That's a milestone in treating rare blood disorders.
Additionally, Novo Nordisk has been in the lead with Mim8, a potential once-weekly or monthly prophylactic treatment for hemophilia A, while concizumab (another treatment from Novo Nordisk) has been recommended by the EMA for approval as a once-daily subcutaneous prophylactic treatment for hemophilia A or B with inhibitors, which could change the management of these conditions.
The surge in biopharmaceutical innovations, including recombinant coagulation factors, gene therapies, and enzyme replacement therapies supports the growth in this market. Moreover, new clinical trials and research regarding new therapies for common and rare bleeding disorders are also projected to drive growth in this market over the next years
Drivers
The increase in the world prevalence of bleeding disorders, especially hemophilia, represents a principal driver of the bleeding disorders treatment market.
Hemophilia A accounts for about 80% of all cases of hemophilia. It afflicts 1 in every 5,000 males born worldwide, and Hemophilia B affects 1 in every 25,000 males. The patients have led to a greatly increased number of patients needing specialized treatment. Moreover, diseases such as Von Willebrand Disease (vWD), which is the most common hereditary bleeding disorder, have a prevalence of about 1 in 1,000 individuals around the world thereby further increasing the markets' potential. With growing patient numbers, there is a significant demand for better and easier treatments to address these lifetime conditions.
Advances in gene therapy and recombinant therapies are increasingly driving the growth of this market in bleeding disorders.
Recent approvals, such as etranacogene dezaparvovec-drlb for Hemophilia B, with long-term efficacy from a single infusion, are revolutionizing treatment choices. For instance, the HOPE-B study, which in December 2023 confirmed the durability and safety of this gene therapy, exemplifies the potential for gene therapy to replace regular infusions and reduce bleeding episodes in hemophilia patients. Similarly, recombinant factor concentrates have enabled more effective treatment of bleeding disorders, helping prevent spontaneous bleeding and improve the quality of life for patients. Such innovations are expected to result in long-term growth in the market.
Market players driving the growth of the Bleeding Disorders Treatment Market through gene therapy and recombinant therapies:
| Company | Key Therapy | Focus Area | Recent Developments |
|---|---|---|---|
|
CSL Behring |
Etranacogene dezaparvovec-drlb (Gene Therapy for Hemophilia B) |
Gene therapy for Hemophilia B |
The HOPE-B study (December 2023) confirmed long-term durability and safety with a one-time infusion. |
|
Takeda |
ADAMTS13, recombinant-krhn (Recombinant Enzyme Replacement Therapy) |
Treatment for Congenital Thrombotic Thrombocytopenic Purpura (cTTP) |
FDA approval in November 2023 for the first recombinant enzyme replacement therapy for cTTP. |
|
Novo Nordisk |
Mim8 (Recombinant Therapy for Hemophilia A) |
Prophylactic treatment for Hemophilia A |
Positive phase 3 results for once-weekly and once-monthly dosing (June 2024). |
|
|
Concizumab (Recombinant Therapy for Hemophilia A or B with inhibitors) |
Prophylactic treatment for Hemophilia A and B with inhibitors |
EMA recommendation for approval (October 2024), a once-daily subcutaneous therapy for patients with inhibitors. |
Restraint
Limited Patient Population Hindering Market Growth of Bleeding Disorders Treatment
One of the biggest challenges facing the treatment market for bleeding disorders is the low number of patients for certain rare conditions, such as congenital thrombotic thrombocytopenic purpura (cTTP) and specific subtypes of Von Willebrand Disease (vWD). Such conditions are very rare; cTTP, for example, affects only 1 in a million. The low number of patients puts a cap on the demand for specialized therapies, hence diminishing the incentive for companies to invest heavily in research and development. This results in slow treatment adoption and market growth.
By Type
Hemophilia A segment dominated the bleeding disorders market with a market share of 55% in 2023. This is because Hemophilia A accounts for the majority at 80% of hemophilia cases globally. With an estimated figure of 1 in 5,000 male births affected, it drives a significant portion of the demand for treatments for bleeding disorders. Still ongoing are the advancements within recombinant therapies, including gene therapies specifically targeted toward Hemophilia A.
Von Willebrand Disease is the fastest-growing segment in this market with the highest CAGR during the forecast period. vWD is the most common inherited bleeding disorder, affecting about 1 in 1,000 people worldwide. Its growth is driven by increased diagnosis rates and better treatment options, especially for those with severe vWD. The growing awareness, improved diagnostic tools, and the introduction of new treatments tailored to vWD are expected to fuel this segment's growth over the forecast period.
By Drug Class
The Recombinant Coagulation Factor Concentrates segment dominated the market with a market share of 40% in 2023. This is because the high efficacy rate, better safety profiles, and ability to avoid bleeding events have made them standard treatments in Hemophilia A and B cases. These products have essentially replaced plasma-derived therapy as a treatment choice primarily because recombinant drugs are less likely to spread blood-borne pathogens; they can offer long-term benefits through fewer injections and are significantly pure.
The desmopressin segment is the fastest growing in the bleeding disorder treatment market, mainly for its increasing use in mild Hemophilia A and Von Willebrand Disease (vWD). Desmopressin is favored because of its affordability, ease of administration, and effectiveness in promoting blood clotting in patients with these milder forms of bleeding disorders. As awareness spreads, its use is being extended, especially in settings where more expensive therapies cannot be accessed.
By End-User
The hospital segment dominated the market with a market share of 50% in 2023. This is generally because the condition is much more complex, and most need hospital-based treatment. All advanced medical structures and specialty care providers are to be found within hospitals in managing treatments for conditions like Hemophilia and vWD through gene therapies, recombinant factor concentrates, and even newer treatments available. In addition, advanced treatment is usually expensive, which makes it more readily available in hospitals where all services are provided.
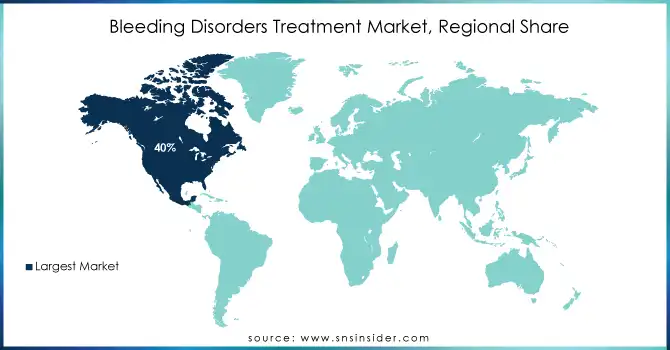
North America dominated the bleeding disorders treatment market with a 40% market share, largely driven by the high prevalence of hemophilia and other bleeding disorders, advanced healthcare infrastructure, and a high adoption rate for treatment. The United States in particular is one of the largest markets because of a large patient population suffering from bleeding disorders. Several top pharmaceutical companies in the United States are involved in research and development, producing next-generation treatments like gene therapies and recombinant factor concentrates. The region also boasts a strong healthcare system, insurance, and easy access to specialized medical centers with comprehensive care for bleeding disorder patients. The approval processes for new treatments by the FDA in North America are much faster than those in other regions. Further, ongoing research and development work in the United States and Canada continue to advance the science behind treatment innovation, especially concerning recombinant therapies and gene therapies.
Asia Pacific is the fastest-growing region in the bleeding disorders treatment market during the forecast period. The region is seeing tremendous growth due to awareness and improvement in healthcare infrastructure as well as rising diagnostic capabilities. In countries like China and India, where immense underserved populations are suffering from bleeding disorders, the market is expanding as better options are becoming available. With improving economic conditions, more patients in these regions are gaining access to advanced therapies such as recombinant coagulation factor concentrates and desmopressin. Also, increasing healthcare expenditures and the attempts to enhance the diagnosis of bleeding disorders are fuelling market growth. Continued rapid adoption of newer therapies, such as gene therapies, should be expected with modernization in countries' healthcare systems and public health initiatives that increase awareness of rare bleeding disorders.
Get Customized Report as per Your Business Requirement - Enquiry Now
Key Market Players:
Biogen Inc. (Eloctate, Alprolix)
CSL Behring (Humate-P, Idelvion)
Pfizer Inc. (BeneFIX, Xyntha)
Novo Nordisk A/S (Novoeight, Refixia)
Grifols S.A. (Alphanate, Fanhdi)
Bayer AG (Kogenate, Jivi)
Octapharma AG (Wilate, Nuwiq)
Sanofi (Altuviiio, Obizur)
Roche (Hemlibra, ACE910)
Takeda Pharmaceutical (Advate, Feiba)
Ferring Pharmaceuticals (Desmopressin, Stimate Nasal Spray)
Baxter International (Obizur, Rixubis)
Amgen Inc. (Investigational therapies)
Alnylam Pharmaceuticals (RNAi-based treatments)
Bristol-Myers Squibb (Clinical-stage therapies)
Janssen Pharmaceuticals (Coagulopathy treatments)
Zydus Lifesciences (Plasma-derived factor concentrates, Generic antifibrinolytics)
Sun Pharmaceuticals (Tranexamic acid, Desmopressin)
AbbVie Inc. (Clotting factor stabilizers, Anticoagulant reversal agents)
Bausch & Lomb (Hemostatic products, Specialty bleeding management solutions)
Key suppliers
key suppliers in the bleeding disorders treatment market can involve raw material providers, manufacturers of specialized proteins and recombinant products, and those supplying critical components for medication development.
Lonza Group AG Suppliers
Thermo Fisher Scientific Inc. Suppliers
Merck KGaA (MilliporeSigma) Suppliers
Cytiva Suppliers
Bio-Rad Laboratories, Inc. Suppliers
Sartorius AG Suppliers
Charles River Laboratories International, Inc. Suppliers
WuXi Biologics Suppliers
Octapharma AG Suppliers
Catalent, Inc. Suppliers
Recent Developments:
June 2024: Novo Nordisk revealed positive outcomes from the phase 3 FRONTIER2 trial, which involved 254 participants aged 12 and older with hemophilia A, both with and without inhibitors. The trial assessed Mim8, an investigational therapy, under once-weekly and once-monthly prophylactic treatment regimens aimed at reducing prolonged and spontaneous bleeding episodes.
October 2024: Novo Nordisk announced that its innovative product, concizumab, received a positive recommendation from the EMA’s Committee for Medicinal Products for Human Use (CHMP). Pending European Commission approval, concizumab would become the first once-daily, subcutaneous prophylactic treatment for hemophilia A or B with inhibitors in patients aged 12 years or older, offering a novel solution for managing these conditions across the European Union.
December 2023: Global biotechnology leader CSL announced promising three-year results from the pivotal HOPE-B study. The findings highlighted the long-term durability and safety of etranacogene dezaparvovec-drlb following a one-time infusion in patients with hemophilia B. This innovative gene therapy demonstrates significant potential for managing the condition effectively.
November 2023: Takeda achieved a milestone with the U.S. FDA approval of ADAMTS13, recombinant-krhn. This marks the first-ever recombinant ADAMTS13 enzyme replacement therapy designed to treat congenital thrombotic thrombocytopenic purpura (cTTP), providing a groundbreaking therapeutic option for patients with this rare blood disorder.
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | US$ 16.35 Billion |
| Market Size by 2032 | US$ 35.17 Billion |
| CAGR | CAGR of 8.91% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | •By Type (Hemophilia A, Hemophilia B, Von Willebrand Disease (vWD), Others) •By Drug Class (Plasma Derived Coagulation Factor Concentrates, Recombinant Coagulation Factor Concentrate, Desmopressin, Antifibrinolytics, Fibrin Sealants, Other Bleeding Disorders Treatment) • By End-Users (Hospitals, Clinics, Research Institutes, Others) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe [Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | Biogen Inc., CSL Behring, Pfizer Inc., Novo Nordisk A/S, Grifols S.A., Bayer AG, Octapharma AG, Sanofi, Roche, Takeda Pharmaceutical, Ferring Pharmaceuticals, Baxter International, Amgen Inc., Alnylam Pharmaceuticals, Bristol-Myers Squibb, Janssen Pharmaceuticals, Zydus Lifesciences, Sun Pharmaceuticals, AbbVie Inc., Bausch & Lomb, and other players. |
| Key Drivers | • The increase in the world prevalence of bleeding disorders, especially hemophilia, represents a principal driver of the bleeding disorders treatment market. • Advances in gene therapy and recombinant therapies are increasingly driving the growth of this market in bleeding disorders. |
| Restraints | • Limited Patient Population Hindering Market Growth of Bleeding Disorders Treatment |
Ans- The Bleeding Disorders Treatment Market was valued at USD 16.35 billion in 2023 and is expected to reach USD 35.17 billion by 2032.
Ans – The CAGR rate of the Bleeding Disorders Treatment Market during 2024-2032 is 8.91%.
Ans- The Hemophilia A segment dominated the market by 55%
Ans- North America held the largest revenue share by 40%.
Ans- Asia Pacific is the fastest-growing Bleeding Disorders Treatment Market region.
1. Introduction
1.1 Market Definition
1.2 Scope (Inclusion and Exclusions)
1.3 Research Assumptions
2. Executive Summary
2.1 Market Overview
2.2 Regional Synopsis
2.3 Competitive Summary
3. Research Methodology
3.1 Top-Down Approach
3.2 Bottom-up Approach
3.3. Data Validation
3.4 Primary Interviews
4. Market Dynamics Impact Analysis
4.1 Market Driving Factors Analysis
4.1.1 Drivers
4.1.2 Restraints
4.1.3 Opportunities
4.1.4 Challenges
4.2 PESTLE Analysis
4.3 Porter’s Five Forces Model
5. Statistical Insights and Trends Reporting
5.1 Market Share by Product Type
5.2 Patient Population Statistics
5.3 Treatment Adoption Rates
5.4 Prevalence and Incidence Rates
5.5 Cost of Treatment
5.6 Insurance Coverage and Access
6. Competitive Landscape
6.1 List of Major Companies, By Region
6.2 Market Share Analysis, By Region
6.3 Product Benchmarking
6.3.1 Product specifications and features
6.3.2 Pricing
6.4 Strategic Initiatives
6.4.1 Marketing and promotional activities
6.4.2 Distribution and Supply Chain Strategies
6.4.3 Expansion plans and new product launches
6.4.4 Strategic partnerships and collaborations
6.5 Technological Advancements
6.6 Market Positioning and Branding
7. Bleeding Disorders Treatment Market Segmentation, by Type
7.1 Chapter Overview
7.2 Hemophilia A
7.2.1 Hemophilia A Market Trends Analysis (2020-2032)
7.2.2 Hemophilia A Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3 Hemophilia B
7.3.1 Hemophilia B Market Trends Analysis (2020-2032)
7.3.2 Hemophilia B Market Size Estimates and Forecasts to 2032 (USD Billion)
7.4 Von Willebrand Disease (vWD)
7.4.1 Von Willebrand Disease (vWD) Market Trends Analysis (2020-2032)
7.4.2 Von Willebrand Disease (vWD) Market Size Estimates and Forecasts to 2032 (USD Billion)
7.5 Others
7.5.1 Others Market Trends Analysis (2020-2032)
7.5.2 Others Market Size Estimates and Forecasts to 2032 (USD Billion)
8. Bleeding Disorders Treatment Market Segmentation, by Drug Class
8.1 Chapter Overview
8.2 Plasma Derived Coagulation Factor Concentrates
8.2.1 Plasma Derived Coagulation Factor Concentrates Market Trends Analysis (2020-2032)
8.2.2 Plasma Derived Coagulation Factor Concentrates Market Size Estimates and Forecasts to 2032 (USD Billion)
8.3 Recombinant Coagulation Factor Concentrate
8.3.1 Recombinant Coagulation Factor Concentrate Market Trends Analysis (2020-2032)
8.3.2 Recombinant Coagulation Factor Concentrate Market Size Estimates and Forecasts to 2032 (USD Billion)
8.4 Desmopressin
8.4.1 Desmopressin Market Trends Analysis (2020-2032)
8.4.2 Desmopressin Market Size Estimates and Forecasts to 2032 (USD Billion)
8.5 Antifibrinolytic
8.5.1 Antifibrinolytic Market Trends Analysis (2020-2032)
8.5.2 Antifibrinolytic Market Size Estimates and Forecasts to 2032 (USD Billion)
8.6 Fibrin Sealants
8.6.1 Fibrin Sealants Market Trends Analysis (2020-2032)
8.6.2 Fibrin Sealants Market Size Estimates and Forecasts to 2032 (USD Billion)
8.7 Other Bleeding Disorders Treatment
8.7.1 Other Bleeding Disorders Treatment Market Trends Analysis (2020-2032)
8.7.2 Other Bleeding Disorders Treatment Market Size Estimates and Forecasts to 2032 (USD Billion)
9. Bleeding Disorders Treatment Market Segmentation, by End User
9.1 Chapter Overview
9.2 Hospitals
9.2.1 Hospitals Market Trends Analysis (2020-2032)
9.2.2 Hospitals Market Size Estimates and Forecasts to 2032 (USD Billion)
9.3 Clinics
9.3.1 Clinics Market Trends Analysis (2020-2032)
9.3.2 Clinics Market Size Estimates and Forecasts to 2032 (USD Billion)
9.4 Research Institute
9.4.1 Research Institute Market Trends Analysis (2020-2032)
9.4.2 Research Institute Market Size Estimates and Forecasts to 2032 (USD Billion)
9.5 Others
9.5.1 Others Market Trends Analysis (2020-2032)
9.5.2 Others Market Size Estimates and Forecasts to 2032 (USD Billion)
10. Regional Analysis
10.1 Chapter Overview
10.2 North America
10.2.1 Trends Analysis
10.2.2 North America Bleeding Disorders Treatment Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.2.3 North America Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.2.4 North America Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.2.5 North America Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.2.6 USA
10.2.6.1 USA Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.2.6.2 USA Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.2.6.3 USA Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.2.7 Canada
10.2.7.1 Canada Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.2.7.2 Canada Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.2.7.3 Canada Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.2.8 Mexico
10.2.8.1 Mexico Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.2.8.2 Mexico Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.2.8.3 Mexico Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.3 Europe
10.3.1 Eastern Europe
10.3.1.1 Trends Analysis
10.3.1.2 Eastern Europe Bleeding Disorders Treatment Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.3.1.3 Eastern Europe Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.1.4 Eastern Europe Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.3.1.5 Eastern Europe Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.3.1.6 Poland
10.3.1.6.1 Poland Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.1.6.2 Poland Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.3.1.6.3 Poland Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.3.1.7 Romania
10.3.1.7.1 Romania Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.1.7.2 Romania Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.3.1.7.3 Romania Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.3.1.8 Hungary
10.3.1.8.1 Hungary Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.1.8.2 Hungary Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.3.1.8.3 Hungary Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.3.1.9 Turkey
10.3.1.9.1 Turkey Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.1.9.2 Turkey Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.3.1.9.3 Turkey Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.3.1.10 Rest of Eastern Europe
10.3.1.10.1 Rest of Eastern Europe Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.1.10.2 Rest of Eastern Europe Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.3.1.10.3 Rest of Eastern Europe Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.3.2 Western Europe
10.3.2.1 Trends Analysis
10.3.2.2 Western Europe Bleeding Disorders Treatment Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.3.2.3 Western Europe Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.2.4 Western Europe Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.3.2.5 Western Europe Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.3.2.6 Germany
10.3.2.6.1 Germany Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.2.6.2 Germany Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.3.2.6.3 Germany Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.3.2.7 France
10.3.2.7.1 France Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.2.7.2 France Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.3.2.7.3 France Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.3.2.8 UK
10.3.2.8.1 UK Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.2.8.2 UK Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.3.2.8.3 UK Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.3.2.9 Italy
10.3.2.9.1 Italy Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.2.9.2 Italy Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.3.2.9.3 Italy Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.3.2.10 Spain
10.3.2.10.1 Spain Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.2.10.2 Spain Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.3.2.10.3 Spain Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.3.2.11 Netherlands
10.3.2.11.1 Netherlands Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.2.11.2 Netherlands Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.3.2.11.3 Netherlands Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.3.2.12 Switzerland
10.3.2.12.1 Switzerland Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.2.12.2 Switzerland Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.3.2.12.3 Switzerland Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.3.2.13 Austria
10.3.2.13.1 Austria Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.2.13.2 Austria Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.3.2.13.3 Austria Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.3.2.14 Rest of Western Europe
10.3.2.14.1 Rest of Western Europe Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.2.14.2 Rest of Western Europe Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.3.2.14.3 Rest of Western Europe Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.4 Asia Pacific
10.4.1 Trends Analysis
10.4.2 Asia Pacific Bleeding Disorders Treatment Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.4.3 Asia Pacific Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.4.4 Asia Pacific Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.4.5 Asia Pacific Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.4.6 China
10.4.6.1 China Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.4.6.2 China Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.4.6.3 China Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.4.7 India
10.4.7.1 India Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.4.7.2 India Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.4.7.3 India Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.4.8 Japan
10.4.8.1 Japan Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.4.8.2 Japan Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.4.8.3 Japan Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.4.9 South Korea
10.4.9.1 South Korea Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.4.9.2 South Korea Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.4.9.3 South Korea Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.4.10 Vietnam
10.4.10.1 Vietnam Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.4.10.2 Vietnam Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.4.10.3 Vietnam Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.4.11 Singapore
10.4.11.1 Singapore Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.4.11.2 Singapore Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.4.11.3 Singapore Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.4.12 Australia
10.4.12.1 Australia Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.4.12.2 Australia Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.4.12.3 Australia Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.4.13 Rest of Asia Pacific
10.4.13.1 Rest of Asia Pacific Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.4.13.2 Rest of Asia Pacific Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.4.13.3 Rest of Asia Pacific Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.5 Middle East and Africa
10.5.1 Middle East
10.5.1.1 Trends Analysis
10.5.1.2 Middle East Bleeding Disorders Treatment Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.5.1.3 Middle East Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.5.1.4 Middle East Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.5.1.5 Middle East Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.5.1.6 UAE
10.5.1.6.1 UAE Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.5.1.6.2 UAE Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.5.1.6.3 UAE Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.5.1.7 Egypt
10.5.1.7.1 Egypt Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.5.1.7.2 Egypt Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.5.1.7.3 Egypt Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.5.1.8 Saudi Arabia
10.5.1.8.1 Saudi Arabia Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.5.1.8.2 Saudi Arabia Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.5.1.8.3 Saudi Arabia Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.5.1.9 Qatar
10.5.1.9.1 Qatar Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.5.1.9.2 Qatar Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.5.1.9.3 Qatar Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.5.1.10 Rest of Middle East
10.5.1.10.1 Rest of Middle East Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.5.1.10.2 Rest of Middle East Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.5.1.10.3 Rest of Middle East Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.5.2 Africa
10.5.2.1 Trends Analysis
10.5.2.2 Africa Bleeding Disorders Treatment Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.5.2.3 Africa Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.5.2.4 Africa Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.5.2.5 Africa Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.5.2.6 South Africa
10.5.2.6.1 South Africa Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.5.2.6.2 South Africa Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.5.2.6.3 South Africa Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.5.2.7 Nigeria
10.5.2.7.1 Nigeria Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.5.2.7.2 Nigeria Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.5.2.7.3 Nigeria Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.5.2.8 Rest of Africa
10.5.2.8.1 Rest of Africa Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.5.2.8.2 Rest of Africa Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.5.2.8.3 Rest of Africa Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.6 Latin America
10.6.1 Trends Analysis
10.6.2 Latin America Bleeding Disorders Treatment Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.6.3 Latin America Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.6.4 Latin America Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.6.5 Latin America Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.6.6 Brazil
10.6.6.1 Brazil Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.6.6.2 Brazil Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.6.6.3 Brazil Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.6.7 Argentina
10.6.7.1 Argentina Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.6.7.2 Argentina Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.6.7.3 Argentina Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.6.8 Colombia
10.6.8.1 Colombia Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.6.8.2 Colombia Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.6.8.3 Colombia Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
10.6.9 Rest of Latin America
10.6.9.1 Rest of Latin America Bleeding Disorders Treatment Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.6.9.2 Rest of Latin America Bleeding Disorders Treatment Market Estimates and Forecasts, by Drug Class (2020-2032) (USD Billion)
10.6.9.3 Rest of Latin America Bleeding Disorders Treatment Market Estimates and Forecasts, by End User (2020-2032) (USD Billion)
11. Company Profiles
11.1 Biogen Inc.
11.1.1 Company Overview
11.1.2 Financial
11.1.3 Products/ Services Offered
11.1.4 SWOT Analysis
11.2 CSL Behring
11.2.1 Company Overview
11.2.2 Financial
11.2.3 Products/ Services Offered
11.2.4 SWOT Analysis
11.3 Pfizer Inc.
11.3.1 Company Overview
11.3.2 Financial
11.3.3 Products/ Services Offered
11.3.4 SWOT Analysis
11.4 Novo Nordisk A/S
11.4.1 Company Overview
11.4.2 Financial
11.4.3 Products/ Services Offered
11.4.4 SWOT Analysis
11.5 Grifols S.A.
11.5.1 Company Overview
11.5.2 Financial
11.5.3 Products/ Services Offered
11.5.4 SWOT Analysis
11.6 Bayer AG
11.6.1 Company Overview
11.6.2 Financial
11.6.3 Products/ Services Offered
11.6.4 SWOT Analysis
11.7 Octapharma AG
11.7.1 Company Overview
11.7.2 Financial
11.7.3 Products/ Services Offered
11.7.4 SWOT Analysis
11.8 Takeda Pharmaceutical
11.8.1 Company Overview
11.8.2 Financial
11.8.3 Products/ Services Offered
11.8.4 SWOT Analysis
11.9 Sanofi
11.9.1 Company Overview
11.9.2 Financial
11.9.3 Products/ Services Offered
11.9.4 SWOT Analysis
11.10 Ferring Pharmaceuticals
11.10.1 Company Overview
11.10.2 Financial
11.10.3 Products/ Services Offered
11.10.4 SWOT Analysis
12. Use Cases and Best Practices
13. Conclusion
An accurate research report requires proper strategizing as well as implementation. There are multiple factors involved in the completion of good and accurate research report and selecting the best methodology to compete the research is the toughest part. Since the research reports we provide play a crucial role in any company’s decision-making process, therefore we at SNS Insider always believe that we should choose the best method which gives us results closer to reality. This allows us to reach at a stage wherein we can provide our clients best and accurate investment to output ratio.
Each report that we prepare takes a timeframe of 350-400 business hours for production. Starting from the selection of titles through a couple of in-depth brain storming session to the final QC process before uploading our titles on our website we dedicate around 350 working hours. The titles are selected based on their current market cap and the foreseen CAGR and growth.
The 5 steps process:
Step 1: Secondary Research:
Secondary Research or Desk Research is as the name suggests is a research process wherein, we collect data through the readily available information. In this process we use various paid and unpaid databases which our team has access to and gather data through the same. This includes examining of listed companies’ annual reports, Journals, SEC filling etc. Apart from this our team has access to various associations across the globe across different industries. Lastly, we have exchange relationships with various university as well as individual libraries.

Step 2: Primary Research
When we talk about primary research, it is a type of study in which the researchers collect relevant data samples directly, rather than relying on previously collected data. This type of research is focused on gaining content specific facts that can be sued to solve specific problems. Since the collected data is fresh and first hand therefore it makes the study more accurate and genuine.
We at SNS Insider have divided Primary Research into 2 parts.
Part 1 wherein we interview the KOLs of major players as well as the upcoming ones across various geographic regions. This allows us to have their view over the market scenario and acts as an important tool to come closer to the accurate market numbers. As many as 45 paid and unpaid primary interviews are taken from both the demand and supply side of the industry to make sure we land at an accurate judgement and analysis of the market.
This step involves the triangulation of data wherein our team analyses the interview transcripts, online survey responses and observation of on filed participants. The below mentioned chart should give a better understanding of the part 1 of the primary interview.

Part 2: In this part of primary research the data collected via secondary research and the part 1 of the primary research is validated with the interviews from individual consultants and subject matter experts.
Consultants are those set of people who have at least 12 years of experience and expertise within the industry whereas Subject Matter Experts are those with at least 15 years of experience behind their back within the same space. The data with the help of two main processes i.e., FGDs (Focused Group Discussions) and IDs (Individual Discussions). This gives us a 3rd party nonbiased primary view of the market scenario making it a more dependable one while collation of the data pointers.
Step 3: Data Bank Validation
Once all the information is collected via primary and secondary sources, we run that information for data validation. At our intelligence centre our research heads track a lot of information related to the market which includes the quarterly reports, the daily stock prices, and other relevant information. Our data bank server gets updated every fortnight and that is how the information which we collected using our primary and secondary information is revalidated in real time.

Step 4: QA/QC Process
After all the data collection and validation our team does a final level of quality check and quality assurance to get rid of any unwanted or undesired mistakes. This might include but not limited to getting rid of the any typos, duplication of numbers or missing of any important information. The people involved in this process include technical content writers, research heads and graphics people. Once this process is completed the title gets uploader on our platform for our clients to read it.
Step 5: Final QC/QA Process:
This is the last process and comes when the client has ordered the study. In this process a final QA/QC is done before the study is emailed to the client. Since we believe in giving our clients a good experience of our research studies, therefore, to make sure that we do not lack at our end in any way humanly possible we do a final round of quality check and then dispatch the study to the client.
By Type
Hemophilia A
Hemophilia B
Von Willebrand Disease (vWD)
Others
By Drug Class
Plasma Derived Coagulation Factor Concentrates
Recombinant Coagulation Factor Concentrate
Desmopressin
Antifibrinolytics
Fibrin Sealants
Other bleeding Disorders Treatment
By End-Users
Hospital
Clinics
Research Institutes
Others
Request for Segment Customization as per your Business Requirement: Segment Customization Request
REGIONAL COVERAGE:
North America
US
Canada
Mexico
Europe
Eastern Europe
Poland
Romania
Hungary
Turkey
Rest of Eastern Europe
Western Europe
Germany
France
UK
Italy
Spain
Netherlands
Switzerland
Austria
Rest of Western Europe
Asia Pacific
China
India
Japan
South Korea
Vietnam
Singapore
Australia
Rest of Asia Pacific
Middle East & Africa
Middle East
UAE
Egypt
Saudi Arabia
Qatar
Rest of the Middle East
Africa
Nigeria
South Africa
Rest of Africa
Latin America
Brazil
Argentina
Colombia
Rest of Latin America
Request for Country Level Research Report: Country Level Customization Request
Available Customization
With the given market data, SNS Insider offers customization to meet the company’s specific needs. The following customization options are available for the report:
Product Analysis
Criss-Cross segment analysis (e.g. Product X Application)
Product Matrix which gives a detailed comparison of the product portfolio of each company
Geographic Analysis
Additional countries in any of the regions
Company Information
Detailed analysis and profiling of additional market players (Up to five)
The Surgical Lights Market Size was valued at USD 2.14 Billion in 2023 and is expected to reach USD 3.05 Billion by 2032 and grow at a CAGR of 4.12% over the forecast period 2024-2032.
The Serum-Free Media Market Size was valued at USD 1.7 billion in 2023 and is expected to grow at a CAGR of 13.0% to reach USD 5.1 billion by 2032.
Fill-Finish Manufacturing Market Size was valued at USD 15.4 Billion in 2023 and is expected to reach USD 33.5 Billion by 2032, growing at a CAGR of 9.1% over the forecast period 2024-2032.
The Mental Health Apps Market Size, valued at USD 6.10 billion in 2023, is projected to reach USD 22.3 billion by 2032, growing at a CAGR of 15.5%.
The Radiation Dose Optimization Software Market was valued at USD 3.80 Billion in 2023 and will reach USD 8.06 Billion by 2032, with a growing CAGR of 8.7% during 2024-2032.
The Diabetic Neuropathy market size was USD 4.28 billion in 2023 and is expected to reach USD 8.18 billion by 2032 and grow at a CAGR of 7.48% over the forecast period of 2024-2032.
Hi! Click one of our member below to chat on Phone