Get More Information on Bionic Eye Market - Request Sample Report
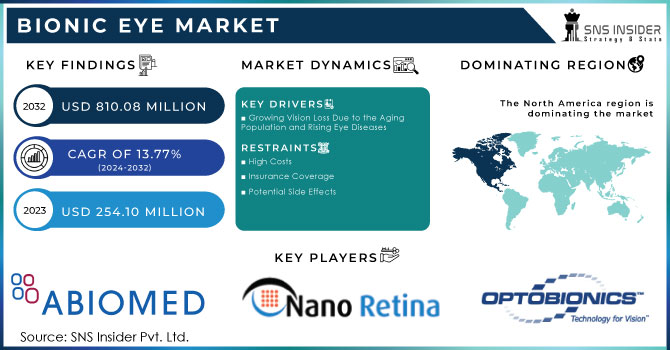
The Bionic Eye Market size was valued at USD 254.10 Million in 2023 and is expected to reach USD 810.08 Million by 2032 and grow at a CAGR of 13.77% over the forecast period 2024-2032.
The Increasing Prevalence of Vision Loss Drives Bionic Eye Market Expansion
The bionic eye market is witnessing rapid growth, driven primarily by the increasing prevalence of vision loss, particularly among aging populations. This trend is further accelerated by the rising incidence of accidents and injuries, leading to a heightened demand for sight restoration solutions. Moreover, advancements in bionic eye technology, including product approvals and intensive research and development efforts, are significantly contributing to the market's expansion. According to the World Health Organization, over 2.2 billion people worldwide were affected by vision impairment or blindness in 2023, with a significant portion suffering from unaddressed or preventable conditions. The positive outcomes of clinical trials involving bionic eyes have further accelerated their adoption among individuals with incurable eye diseases and permanent vision loss, demonstrating the safety, reliability, and long-term efficacy of this technology.
Market Insights on Vision Loss and Eye Disorders
The demand for bionic eyes is also driven by the increasing prevalence of eye disorders such as retinitis pigmentosa (RP) and age-related macular degeneration. According to the National Eye Institute, retinitis pigmentosa is a rare genetic disorder affecting approximately one in 4,000 people worldwide. Additionally, the Bright Focus Foundation reports that approximately 11 million individuals in the United States are affected by age-related macular degeneration, with this number projected to double by 2050. Globally, the foundation estimates that the number of people with macular degeneration will reach 288 million by 2040, up from 196 million in 2020.
Technological Advancements Fuel Market Growth
The bionic eye market is experiencing growth as technological advancements and research continue to evolve. The development of more affordable and accessible bionic eye solutions is driving their adoption. Additionally, rising investments in research focused on sight restoration are fueling market growth. The growing number of individuals with complete or partial blindness and the increasing prevalence of eye disorders, such as those related to diabetes, are further contributing to the demand for bionic eye technology.
Regulatory Approvals and Global Investments
Regulatory approvals and guidelines play a crucial role in ensuring the safety and efficacy of bionic eye devices. Governments worldwide are also investing in research and development to support the advancement of bionic eye technology. For instance, Bionic Vision Technologies (BVT), an Australian company, announced on January 16, 2022, that its Bionic Eye System had received Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA). This designation is a significant milestone, indicating the FDA's belief that the device has the potential to treat a serious condition or fill a significant unmet medical need. This achievement marks Australia's first bionic eye system to receive such recognition from the U.S. FDA, highlighting the country's advancements in medical technology and innovation.
Innovative Electrode Implants and Bionic Lenses
The introduction of new electrode implants has the potential to provide more natural vision for patients, driving significant market growth. Additionally, the development of bionic lenses that can replace natural lenses and restore vision at all distances represents a major breakthrough. However, while the bionic eye market is growing, it faces challenges such as the high costs associated with these devices, which can limit accessibility. Potential side effects, such as skin reactions, may also hinder adoption. Nevertheless, ongoing research and technological advancements are addressing these challenges, creating new opportunities for market expansion. As research continues and technology improves, bionic eyes have the potential to transform the lives of millions of people with vision impairment.
Drivers
Growing Vision Loss Due to the Aging Population and Rising Eye Diseases:
The increasing prevalence of vision loss, driven by an aging population and rising incidence of eye diseases, is a primary driver. Technological advancements, such as improved electrode implants and the development of bionic lenses, are making these devices more effective and accessible. Positive outcomes from clinical trials have demonstrated the safety, reliability, and efficacy of bionic eyes, boosting their adoption. Government support, including favorable regulations and policies, is fostering market growth by facilitating the development and approval of these devices. Investments in research and development are contributing to market innovation and expansion by driving advancements in bionic eye technology. Rising awareness among patients and healthcare providers is also driving demand, as more individuals become aware of the potential benefits of these devices. Ultimately, the ability of bionic eyes to restore vision and improve the quality of life for individuals with vision impairment is a significant driver for the market.
Restraints
High Costs:
The high cost associated with bionic eye devices can limit accessibility, particularly for individuals with limited financial resources.
Insurance Coverage:
Many health insurance plans do not fully cover the cost of bionic eye procedures, making them unaffordable for some patients.
Potential Side Effects:
Bionic eye implants may be associated with potential side effects, such as skin reactions or discomfort, which could deter some individuals from considering the procedure.
By Type
The external eye segment dominated the bionic eye market, capturing over 60% of the revenue in 2023. This dominance stems from its ability to enhance visual experiences. Market players are actively investing in technological advancements to improve patient outcomes, further solidifying the segment's position. The external eye segment is poised for the fastest growth, projected to expand at a CAGR of 13.6% during the forecast period. This rapid growth is attributed to several factors. Firstly, the segment offers superior visual experiences compared to other options. Secondly, ongoing technological innovations from various industry competitors are driving down procedure costs, making them more accessible to a wider patient population. These combined factors are expected to fuel significant market expansion in the coming years.
By End-use
The hospital segment dominated the bionic eye market, accounting for approximately 45% of the revenue in 2023, and is expected to maintain its lead throughout the forecast period. Hospitals provide the initial point of care, staffed with experienced ophthalmologists and advanced equipment to ensure efficient patient treatment. Expanding medical coverage and successful surgical outcomes are driving an increase in surgical procedures, contributing to the segment's growth. Meanwhile, the outpatient facilities segment is poised for the fastest growth, projected to expand at a CAGR of 13.7%. This surge is attributed to a growing preference for outpatient settings over hospitals. Collaborations between hospitals and product developers for clinical trials have significantly accelerated the segment's development. These factors collectively position outpatient facilities as a rapidly expanding segment within the market.
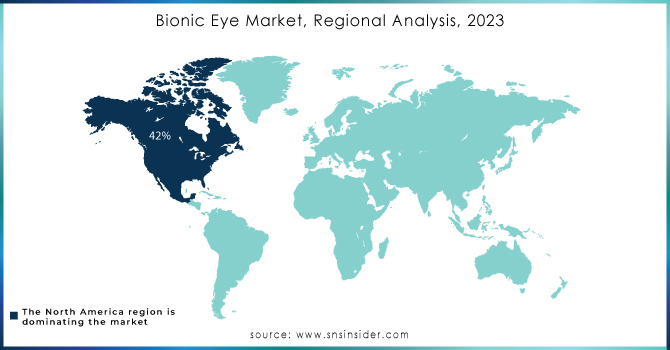
North America dominated the bionic eye market, capturing approximately 42% of the market share in 2023, and is expected to maintain its lead throughout the forecast period. The region's favorable reimbursement policies, well-developed healthcare infrastructure, and robust research activities contribute to its dominance. Technological advancements, such as overcoming the challenges of printed electronics on curved surfaces, are driving significant market growth. In 2018, a collaborative effort between researchers from Stanford University and Israel, funded by the U.S. National Institutes of Health and the U.S. Air Force, focused on developing a solar-powered retina to treat blindness caused by retinitis pigmentosa.
Asia Pacific is poised for the fastest growth, projected to expand at a CAGR of 15.5% during the forecast period. The region's rapid growth is fueled by the accelerated pace at which companies are introducing innovative bionic eye implants. In January 2020, Bionic Vision Technologies, Australia, announced the results of a pilot study conducted on patients with late-stage retinitis pigmentosa using its Gen3 bionic eye system.
Need any customization research on Bionic Eye Market - Enquiry Now
The Major Players are Optobionics Corporation, Monash Vision Group, Nano Retina Ltd., ABIOMED, AAV Media, LLC., NEOSTRATA, Nidek Co. Ltd., Integrated Bionic Microsystems Laboratory, Pixium Vision, Second Sight Medical Products LLC, Biomedical Technologies S.L., MetaModal LLC, THE BIONIC EYE, Bionic Vision Technologies, and others playres
Recent Developments
Second Sight Medical Products:
March 2024: Announced the launch of the Orion Visual Cortical Prosthesis System, designed to enhance visual perception in patients with severe vision loss.
Bionic Vision Technologies:
June 2024: Revealed advancements in their bionic eye prototype, featuring improved electrode implants for better visual acuity and stability.
Pixium Vision:
April 2024: Released updated clinical trial results showing significant improvements in visual outcomes with their PRIMA bionic vision system.
Eyre Bioengineering:
July 2024: Introduced a new generation of bionic lenses, incorporating advanced materials for greater comfort and clarity.
Implantable Bionic Eyes Inc.:
February 2024: Completed a successful pilot study for their next-generation bionic eye device, demonstrating enhanced functionality and user satisfaction.
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | US$ 254.10 million |
| Market Size by 2032 | US$ 810.08 million |
| CAGR | CAGR of 13.77% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Type (External Eye, Implanted Eye) • By End-use (Hospitals, Outpatient Facilities, Research and Manufacturing) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | Optobionics Corporation, Monash Vision Group, Nano Retina Ltd., ABIOMED, AAV Media, LLC., NEOSTRATA, Nidek Co. Ltd., Integrated Bionic Microsystems Laboratory, Pixium Vision, Second Sight Medical Products LLC, Biomedical Technologies S.L., MetaModal LLC, THE BIONIC EYE, Bionic Vision Technologies and others. |
| Key Drivers | • Growing Vision Loss Due to the Aging Population and Rising Eye Diseases Fuels the Bionic Eyes Market |
| Restraints | • The high cost associated with bionic eye devices can limit accessibility, particularly for individuals with limited financial resources. • Many health insurance plans do not fully cover the cost of bionic eye procedures, making them unaffordable for some patients. • Bionic eye implants may be associated with potential side effects, such as skin reactions or discomfort, which could deter some individuals from considering the procedure. |
Ans: The estimated compound annual growth rate is 13.77% during the forecast period for the Bionic Eye market.
Ans: The projected market value of the Bionic Eye market is estimated at USD 254.10 million in 2023 and is expected to reach USD 810.08 million by 2032.
Ans: Growing vision loss due to the aging population and rising eye diseases fuels the bionic eyes market.
Ans: The high cost associated with bionic eye devices can limit accessibility, particularly for individuals with limited financial resources.
Ans: North America is the dominating region with a 42% market share in the Bionic Eye market.
Table of Contents
1. Introduction
1.1 Market Definition
1.2 Scope (Inclusion and Exclusions)
1.3 Research Assumptions
2. Executive Summary
2.1 Market Overview
2.2 Regional Synopsis
2.3 Competitive Summary
3. Research Methodology
3.1 Top-Down Approach
3.2 Bottom-up Approach
3.3. Data Validation
3.4 Primary Interviews
4. Market Dynamics Impact Analysis
4.1 Market Driving Factors Analysis
4.1.2 Drivers
4.1.2 Restraints
4.1.3 Opportunities
4.1.4 Challenges
4.2 PESTLE Analysis
4.3 Porter’s Five Forces Model
5. Statistical Insights and Trends Reporting
5.1 Incidence and Prevalence (2023)
5.2 Prescription Trends, (2023), by Region
5.3 Device Volume, by Region (2020-2032)
5.4 Healthcare Spending, by Region, (Government, Commercial, Private, Out-of-Pocket), 2023
6. Competitive Landscape
6.1 List of Major Companies, By Region
6.2 Market Share Analysis, By Region
6.3 Product Benchmarking
6.3.1 Product specifications and features
6.3.2 Pricing
6.4 Strategic Initiatives
6.4.1 Marketing and promotional activities
6.4.2 Distribution and Supply Chain Strategies
6.4.3 Expansion plans and new product launches
6.4.4 Strategic partnerships and collaborations
6.5 Technological Advancements
6.6 Market Positioning and Branding
7. Bionic Eye Market Segmentation, by Type
7.1 Chapter Overview
7.2 External Eye
7.2.1 External Eye Market Trends Analysis (2020-2032)
7.2.2 External Eye Market Size Estimates and Forecasts to 2032 (USD Million)
7.3 Implanted Eye
7.3.1 Implanted Eye Market Trends Analysis (2020-2032)
7.3.2 Implanted Eye Market Size Estimates and Forecasts to 2032 (USD Million)
8. Bionic Eye Market Segmentation, by End-use
8.1 Chapter Overview
8.2 Hospitals
8.2.1 Hospitals Market Trends Analysis (2020-2032)
8.2.2 Hospitals Market Size Estimates and Forecasts to 2032 (USD Million)
8.3 Outpatient Facilities
8.3.1 Outpatient Facilities Market Trends Analysis (2020-2032)
8.3.2 Outpatient Facilities Market Size Estimates and Forecasts to 2032 (USD Million)
8.4 Research and Manufacturing
8.4.1 Research and Manufacturing Market Trends Analysis (2020-2032)
8.4.2 Research and Manufacturing Market Size Estimates and Forecasts to 2032 (USD Million)
9. Regional Analysis
9.1 Chapter Overview
9.2 North America
9.2.1 Trends Analysis
9.2.2 North America Bionic Eye Market Estimates and Forecasts, by Country (2020-2032) (USD Million)
9.2.3 North America Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.2.4 North America Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.2.5 USA
9.2.5.1 USA Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.2.5.2 USA Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.2.6 Canada
9.2.6.1 Canada Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.2.6.2 Canada Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.2.7 Mexico
9.2.7.1 Mexico Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.2.7.2 Mexico Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.3 Europe
9.3.1 Eastern Europe
9.3.1.1 Trends Analysis
9.3.1.2 Eastern Europe Bionic Eye Market Estimates and Forecasts, by Country (2020-2032) (USD Million)
9.3.1.3 Eastern Europe Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.3.1.4 Eastern Europe Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.3.1.5 Poland
9.3.1.5.1 Poland Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.3.1.5.2 Poland Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.3.1.6 Romania
9.3.1.6.1 Romania Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.3.1.6.2 Romania Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.3.1.7 Hungary
9.3.1.7.1 Hungary Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.3.1.7.2 Hungary Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.3.1.8 Turkey
9.3.1.8.1 Turkey Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.3.1.8.2 Turkey Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.3.1.9 Rest of Eastern Europe
9.3.1.9.1 Rest of Eastern Europe Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.3.1.9.2 Rest of Eastern Europe Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.3.2 Western Europe
9.3.2.1 Trends Analysis
9.3.2.2 Western Europe Bionic Eye Market Estimates and Forecasts, by Country (2020-2032) (USD Million)
9.3.2.3 Western Europe Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.3.2.4 Western Europe Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.3.2.5 Germany
9.3.2.5.1 Germany Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.3.2.5.2 Germany Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.3.2.6 France
9.3.2.6.1 France Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.3.2.6.2 France Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.3.2.7 UK
9.3.2.7.1 UK Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.3.2.7.2 UK Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.3.2.8 Italy
9.3.2.8.1 Italy Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.3.2.8.2 Italy Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.3.2.9 Spain
9.3.2.9.1 Spain Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.3.2.9.2 Spain Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.3.2.10 Netherlands
9.3.2.10.1 Netherlands Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.3.2.10.2 Netherlands Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.3.2.11 Switzerland
9.3.2.11.1 Switzerland Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.3.2.11.2 Switzerland Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.3.2.12 Austria
9.3.2.12.1 Austria Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.3.2.12.2 Austria Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.3.2.13 Rest of Western Europe
9.3.2.13.1 Rest of Western Europe Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.3.2.13.2 Rest of Western Europe Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.4 Asia Pacific
9.4.1 Trends Analysis
9.4.2 Asia Pacific Bionic Eye Market Estimates and Forecasts, by Country (2020-2032) (USD Million)
9.4.3 Asia Pacific Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.4.4 Asia Pacific Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.4.5 China
9.4.5.1 China Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.4.5.2 China Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.4.6 India
9.4.5.1 India Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.4.5.2 India Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.4.5 Japan
9.4.5.1 Japan Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.4.5.2 Japan Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.4.6 South Korea
9.4.6.1 South Korea Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.4.6.2 South Korea Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.4.7 Vietnam
9.4.7.1 Vietnam Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.2.7.2 Vietnam Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.4.8 Singapore
9.4.8.1 Singapore Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.4.8.2 Singapore Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.4.9 Australia
9.4.9.1 Australia Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.4.9.2 Australia Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.4.10 Rest of Asia Pacific
9.4.10.1 Rest of Asia Pacific Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.4.10.2 Rest of Asia Pacific Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.5 Middle East and Africa
9.5.1 Middle East
9.5.1.1 Trends Analysis
9.5.1.2 Middle East Bionic Eye Market Estimates and Forecasts, by Country (2020-2032) (USD Million)
9.5.1.3 Middle East Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.5.1.4 Middle East Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.5.1.5 UAE
9.5.1.5.1 UAE Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.5.1.5.2 UAE Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.5.1.6 Egypt
9.5.1.6.1 Egypt Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.5.1.6.2 Egypt Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.5.1.7 Saudi Arabia
9.5.1.7.1 Saudi Arabia Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.5.1.7.2 Saudi Arabia Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.5.1.8 Qatar
9.5.1.8.1 Qatar Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.5.1.8.2 Qatar Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.5.1.9 Rest of Middle East
9.5.1.9.1 Rest of Middle East Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.5.1.9.2 Rest of Middle East Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.5.2 Africa
9.5.2.1 Trends Analysis
9.5.2.2 Africa Bionic Eye Market Estimates and Forecasts, by Country (2020-2032) (USD Million)
9.5.2.3 Africa Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.5.2.4 Africa Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.5.2.5 South Africa
9.5.2.5.1 South Africa Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.5.2.5.2 South Africa Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.5.2.6 Nigeria
9.5.2.6.1 Nigeria Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.5.2.6.2 Nigeria Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.6 Latin America
9.6.1 Trends Analysis
9.6.2 Latin America Bionic Eye Market Estimates and Forecasts, by Country (2020-2032) (USD Million)
9.6.3 Latin America Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.6.4 Latin America Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.6.5 Brazil
9.6.5.1 Brazil Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.6.5.2 Brazil Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.6.6 Argentina
9.6.6.1 Argentina Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.6.6.2 Argentina Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.6.7 Colombia
9.6.7.1 Colombia Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.6.7.2 Colombia Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
9.6.8 Rest of Latin America
9.6.8.1 Rest of Latin America Bionic Eye Market Estimates and Forecasts, by Type (2020-2032) (USD Million)
9.6.8.2 Rest of Latin America Bionic Eye Market Estimates and Forecasts, by End-use (2020-2032) (USD Million)
10. Company Profiles
10.1 Optobionics Corporation
10.1.1 Company Overview
10.1.2 Financial
10.1.3 Products/ Services Offered
110.1.4 SWOT Analysis
10.2 Monash Vision Group
10.2.1 Company Overview
10.2.2 Financial
10.2.3 Products/ Services Offered
10.2.4 SWOT Analysis
10.3 Nano Retina Ltd.
10.3.1 Company Overview
10.3.2 Financial
10.3.3 Products/ Services Offered
10.3.4 SWOT Analysis
10.4 ABIOMED
10.4.1 Company Overview
10.4.2 Financial
10.4.3 Products/ Services Offered
10.4.4 SWOT Analysis
10.5 AAV Media, LLC.
10.5.1 Company Overview
10.5.2 Financial
10.5.3 Products/ Services Offered
10.5.4 SWOT Analysis
10.6 NEOSTRATA
10.6.1 Company Overview
10.6.2 Financial
10.6.3 Products/ Services Offered
10.6.4 SWOT Analysis
10.7 Nidek Co. Ltd.
10.7.1 Company Overview
10.7.2 Financial
10.7.3 Products/ Services Offered
10.7.4 SWOT Analysis
10.8 Integrated Bionic Microsystems Laboratory
10.8.1 Company Overview
10.8.2 Financial
10.8.3 Products/ Services Offered
10.8.4 SWOT Analysis
10.9 Pixium Vision
10.9.1 Company Overview
10.9.2 Financial
10.9.3 Products/ Services Offered
10.9.4 SWOT Analysis
10.10 Second Sight Medical Products LLC
10.9.1 Company Overview
10.9.2 Financial
10.9.3 Products/ Services Offered
10.9.4 SWOT Analysis
11. Use Cases and Best Practices
12. Conclusion
An accurate research report requires proper strategizing as well as implementation. There are multiple factors involved in the completion of good and accurate research report and selecting the best methodology to compete the research is the toughest part. Since the research reports we provide play a crucial role in any company’s decision-making process, therefore we at SNS Insider always believe that we should choose the best method which gives us results closer to reality. This allows us to reach at a stage wherein we can provide our clients best and accurate investment to output ratio.
Each report that we prepare takes a timeframe of 350-400 business hours for production. Starting from the selection of titles through a couple of in-depth brain storming session to the final QC process before uploading our titles on our website we dedicate around 350 working hours. The titles are selected based on their current market cap and the foreseen CAGR and growth.
The 5 steps process:
Step 1: Secondary Research:
Secondary Research or Desk Research is as the name suggests is a research process wherein, we collect data through the readily available information. In this process we use various paid and unpaid databases which our team has access to and gather data through the same. This includes examining of listed companies’ annual reports, Journals, SEC filling etc. Apart from this our team has access to various associations across the globe across different industries. Lastly, we have exchange relationships with various university as well as individual libraries.

Step 2: Primary Research
When we talk about primary research, it is a type of study in which the researchers collect relevant data samples directly, rather than relying on previously collected data. This type of research is focused on gaining content specific facts that can be sued to solve specific problems. Since the collected data is fresh and first hand therefore it makes the study more accurate and genuine.
We at SNS Insider have divided Primary Research into 2 parts.
Part 1 wherein we interview the KOLs of major players as well as the upcoming ones across various geographic regions. This allows us to have their view over the market scenario and acts as an important tool to come closer to the accurate market numbers. As many as 45 paid and unpaid primary interviews are taken from both the demand and supply side of the industry to make sure we land at an accurate judgement and analysis of the market.
This step involves the triangulation of data wherein our team analyses the interview transcripts, online survey responses and observation of on filed participants. The below mentioned chart should give a better understanding of the part 1 of the primary interview.

Part 2: In this part of primary research the data collected via secondary research and the part 1 of the primary research is validated with the interviews from individual consultants and subject matter experts.
Consultants are those set of people who have at least 12 years of experience and expertise within the industry whereas Subject Matter Experts are those with at least 15 years of experience behind their back within the same space. The data with the help of two main processes i.e., FGDs (Focused Group Discussions) and IDs (Individual Discussions). This gives us a 3rd party nonbiased primary view of the market scenario making it a more dependable one while collation of the data pointers.
Step 3: Data Bank Validation
Once all the information is collected via primary and secondary sources, we run that information for data validation. At our intelligence centre our research heads track a lot of information related to the market which includes the quarterly reports, the daily stock prices, and other relevant information. Our data bank server gets updated every fortnight and that is how the information which we collected using our primary and secondary information is revalidated in real time.

Step 4: QA/QC Process
After all the data collection and validation our team does a final level of quality check and quality assurance to get rid of any unwanted or undesired mistakes. This might include but not limited to getting rid of the any typos, duplication of numbers or missing of any important information. The people involved in this process include technical content writers, research heads and graphics people. Once this process is completed the title gets uploader on our platform for our clients to read it.
Step 5: Final QC/QA Process:
This is the last process and comes when the client has ordered the study. In this process a final QA/QC is done before the study is emailed to the client. Since we believe in giving our clients a good experience of our research studies, therefore, to make sure that we do not lack at our end in any way humanly possible we do a final round of quality check and then dispatch the study to the client.
Key Segments:
By Type
External Eye
Implanted Eye
By End-use
Hospitals
Outpatient Facilities
Research and Manufacturing
Request for Segment Customization as per your Business Requirement: Segment Customization Request
Regional Coverage:
North America
US
Canada
Mexico
Europe
Eastern Europe
Poland
Romania
Hungary
Turkey
Rest of Eastern Europe
Western Europe
Germany
France
UK
Italy
Spain
Netherlands
Switzerland
Austria
Rest of Western Europe
Asia Pacific
China
India
Japan
South Korea
Vietnam
Singapore
Australia
Rest of Asia Pacific
Middle East & Africa
Middle East
UAE
Egypt
Saudi Arabia
Qatar
Rest of Middle East
Africa
Nigeria
South Africa
Rest of Africa
Latin America
Brazil
Argentina
Colombia
Rest of Latin America
Request for Country Level Research Report: Country Level Customization Request
Available Customization
With the given market data, SNS Insider offers customization as per the company’s specific needs. The following customization options are available for the report:
Product Analysis
Criss-Cross segment analysis (e.g. Product X Application)
Product Matrix which gives a detailed comparison of product portfolio of each company
Geographic Analysis
Additional countries in any of the regions
Company Information
Detailed analysis and profiling of additional market players (Up to five)
Synthetic Biology Market Size was valued at USD 12.5 billion in 2023 and is expected to reach USD 60.4 billion by 2032, growing at a CAGR of 19.1% over the forecast period 2024-2032.
Ethylene Oxide (EtO) Sterilization Services Market was valued at USD 4.7 billion in 2023 and is expected to reach USD 10.55 billion by 2032, growing at a CAGR of 10.52% over the forecast period 2024-2032.
The Urinary Catheters Market size was USD 5.80 billion in 2023 and is estimated to reach USD 9.50 billion by 2032 with a growing CAGR of 5.68% by 2024-2032.
The Medical Device Cleaning Market size was valued at USD 21.74 billion in 2023 and is expected to reach USD 51.96 billion by 2032, growing at a CAGR of 10.22% from 2024-2032.
The Empty IV Bags Market size was valued at USD 4.44 Billion in 2023 and is expected to reach USD 8.86 Billion by 2032 and grow at a CAGR of 7.31% over the forecast period 2024-2032.
The ECG Equipment and Management Systems Market size at USD 6.9 billion in 2023, with a projected CAGR of 6.2% to reach USD 11.32 billion by 2031.
Hi! Click one of our member below to chat on Phone