The Assisted Reproductive Technology (ART) Market was valued at USD 28.33 billion in 2023 and is projected to reach USD 55.09 billion by 2032, growing at a CAGR of 7.68%. This report offers insights into the increasing incidence of infertility and geographic trends in ART procedures, as well as success rates by treatment. The report also addresses the most recent technological developments in ART, with a focus on innovations that are enhancing treatment results. It also discusses demographic and societal changes, including increased childbearing delay and increased awareness of fertility preservation, that are fueling demand for ART services worldwide.
Get more information on Assisted Reproductive Technology Market - Request Sample Report
Drivers
Increasing Awareness of Fertility Treatments and Continuous Advancements in Technology.
As increasing numbers of individuals and couples face infertility, they are turning to ART alternatives, driving demand for treatments such as IVF, ICSI, and egg/sperm cryopreservation. Specifically, advances in technology such as genetic screening, embryo freezing, and better IVF culture media have improved treatment success rates considerably, increasing the effectiveness and availability of ART. An ASRM study puts almost 1 in 8 couples at risk of being infertile, and therefore, there is an increased worldwide demand for ART procedures. Greater acceptance of ART by society coupled with more widespread media coverage further diminished the taboo associated with the use of assisted reproductive techniques. The popularity of fertility preservation treatments like egg freezing has also widened the market base. With advancing technology, ART procedures are becoming less invasive, more affordable, and have greater success rates, which also fuels the market.
Restraints
The high cost of treatment and the limited insurance coverage available for fertility treatments.
The cost of an IVF cycle can be between USD 10,000 and USD 15,000, excluding other costs such as medication, diagnostics, or freezing of embryos. In most nations, fertility services are not included in basic health insurance policies and are thus unaffordable for the majority of the population. This economic hurdle deters most prospective patients from seeking ART, especially in developing countries where proper medical care may be scarce. For example, in the US, even with the development of ART, most insurance policies do not include full coverage for fertility procedures, resulting in out-of-pocket costs that many families cannot meet. Although some nations, like Denmark and Belgium, have comprehensive coverage of ART, the absence of universal access on an international scale is a key issue to market growth on a broader scale.
Opportunities
The expansion of fertility services and increasing government support.
Governments in most nations are acknowledging the significance of fertility treatments and are starting to provide more assistance through healthcare reforms, subsidies, or inclusion in public insurance programs. For example, nations such as Israel and France offer government-subsidized ART treatments, which are made available to a larger population. Furthermore, the emerging trend of fertility tourism offers a special opportunity in that people from nations with limited access to ART are going to countries that have advanced fertility services at affordable rates. Additionally, the increasing use of fertility preservation techniques like sperm and egg banking for people who postpone childbearing for professional or personal reasons increases market growth even further. The increase in fertility clinics, particularly in the newly industrializing markets of India and China, is generating greater demand for ART therapies, propelling market growth. Advances in technologies also present chances for creating low-cost, minimal-invasive forms of ART technology, stimulating future adoption.
Challenges
The ethical concerns surrounding reproductive technologies and regulatory barriers in different regions.
The moral controversy surrounding ART, especially about embryo selection, gene editing, and the use of donor eggs and sperm, has resulted in different regulations in different countries. In certain countries, ART procedures are strictly regulated or prohibited, constraining the potential of the market in those countries. For instance, Germany and Italy have very strict legislation around ART that limits access to some procedures, including the utilization of donor eggs or embryos. Such regulatory barriers can be limiting for both healthcare providers and patients, making it difficult for ART to be used on a broad scale. Also, public debates regarding the morality of gene editing and genetic screening technologies like CRISPR add to the difficulties faced by the market. Others have argued that the potential for "designer babies" may pose ethical concerns, making it difficult for the world to embrace sophisticated ART practices. These ethical and regulatory issues, if not properly addressed, may stifle the growth of the market and limit the advancement of new ART therapies.
By Type
In 2023, the In-vitro fertilization (IVF) category led the Assisted Reproductive Technology (ART) market globally, capturing a massive 92.1% of the overall revenue. IVF has emerged as the go-to form of assisted reproduction because of its high success rate and prevalence across the globe. The expansion of the IVF segment is driven by factors such as technological improvements in reproductive technology, advances in embryo culture technology, and greater knowledge of genetic screening. The fact that IVF can treat a broad array of infertility issues, including male infertility, tubal issues, and unexplained infertility, has made it the preferred option for most couples desiring fertility treatment. The significant prevalence of IVF is also to be understood by the growing awareness among the population for the treatment and increased acceptability in diverse populations.
Artificial Insemination (AI) is the most rapidly expanding category in the ART market. AI is less invasive and affordable than IVF, hence a more attractive choice for increasing numbers of patients looking for fertility therapy. Greater accessibility of AI and growing awareness of its advantages are driving its fast expansion. It is especially in vogue for mild male infertility, the use of donor sperm, or when couples opt for a simpler procedure. As more people become aware of the advantages of AI, its usage is going to continue growing, offering a worthwhile alternative to IVF for those who need affordable fertility solutions.
By End-use
In 2023, the fertility clinics & other settings segment held the largest share of the global ART market, generating over 80.2% of the total revenue. Fertility clinics have been the major setting for ART treatments for many years, providing specialized treatment and customized fertility services that are critical to success in reproductive technology. Fertility clinics offer sophisticated diagnostic equipment, individualized treatment plans, and a caring environment for patients who are undergoing ART procedures. The dominance of fertility clinics is also driven by the growing demand for ART, as well as the growing number of fertility treatment options available to patients. The presence of established and expert-run clinics has increased the accessibility and efficacy of ART services. Fertility clinics are likely to maintain their dominant position during the forecast period, based on their expertise, specialized care, and increasing patient demand.
The hospital and others category is the fastest-growing end-use segment of the ART market. Hospitals are now providing ART treatments as part of their services on fertility, with patients enjoying the additional benefit of being offered comprehensive healthcare in one location. Hospital-based ART services have grown mainly because reproductive medicine is increasingly being integrated into mainstream healthcare, which allows these services to be made more accessible to a wider population.
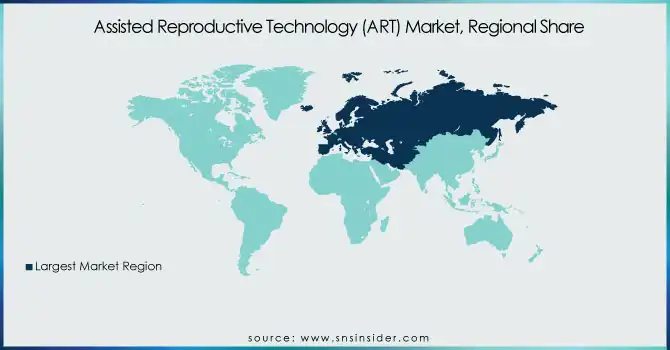
Europe was the leading region in the Assisted Reproductive Technology (ART) market, capturing the highest share as a result of well-developed healthcare infrastructure, favorable government policies, and rising awareness about fertility treatments. The UK, Spain, and Germany are among the leading nations to adopt ART, with well-established fertility centers and a high success rate of the procedure. Europe is also favored by good insurance coverage and publicly funded ART programs, which increase access to treatment. For instance, in France and Denmark, ART treatments are extensively covered by national healthcare programs, leading to increased adoption rates. In addition, there has been an increase in fertility tourism in Europe, with neighboring countries' patients making journeys for ART care because of the good standards of care and legislation that provide for a range of ART procedures. Apart from IVF and ICSI, Europe has also witnessed development in newer ART technologies like genetic screening and embryo freezing, which has further established the continent as the global leader in the ART market.
Need any customization research on Assisted Reproductive Technology Market - Enquiry Now
Key Players
Cosmos Biomedical Ltd. - Sperm Processing Kits, Embryo Transfer Catheters
Microm U.K. Ltd. - Embryo Culture Media, IVF Laboratory Equipment
CooperSurgical, Inc. - Gavi IVF System, EmbryoScope, Fertility Preservation Devices
FUJIFILM Irvine Scientific - ART Culture Media, Oocyte Handling Products
Cryolab Ltd. - Cryopreservation Systems, Cryogenic Vials
Vitrolife AB - Embryo Culture Media, IVF Consumables
European Sperm Bank - Donor Sperm, Sperm Cryopreservation Kits
Bloom IVF Centre - IVF Services, Embryo Culture Media
Merck KGaA - Oocyte Cryopreservation Products, IVF Media
Ferring B.V. - Ovarian Stimulation Products, ART Drugs
Hamilton Thorne, Inc. - CASA (Computer Assisted Semen Analysis) Systems, Micromanipulation Systems
Nikon Corporation - Inverted Microscopes for IVF, Imaging Systems
Nidacon International AB - Sperm Processing and Freezing Products, IVF Consumables
Laboratoire CCD - Sperm Preparation Media, IVF Kits
Esco Micro Pte. Ltd. - IVF Incubators, Sperm Sorting Systems
Recent Developments
In Feb 2025, GenPrime, a prominent network of fertility clinics in Asia and North America, formed a strategic partnership with Genea Fertility, a leading Australian fertility service provider since 1986. This collaboration aims to enhance fertility treatment services and elevate the quality of care for patients in Thailand and Southeast Asia, offering world-class treatment options.
In Nov 2024, SpOvum launched an AI-powered platform designed to enhance patient support in assisted reproductive technology (ART). The platform delivers personalized, fact-based information tailored to individual ART concerns by utilizing indexed medical guides to provide accurate, customized responses.
| Report Attributes | Details |
| Market Size in 2023 | USD 28.33 billion |
| Market Size by 2032 | USD 55.09 Billion |
| CAGR | CAGR of 7.68% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Type [In-vitro fertilization (IVF) (Fresh Donor, Frozen Donor, Fresh Non-Donor, Frozen Non-Donor), Artificial Insemination (Intrauterine Insemination, Intracervical Insemination, Intravaginal Insemination, Intratubal Insemination)] • By End-use [Fertility Clinics & other settings, Hospitals and others] |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | Cosmos Biomedical Ltd., Microm U.K. Ltd., CooperSurgical, Inc., FUJIFILM Irvine Scientific, Cryolab Ltd., Vitrolife AB, European Sperm Bank, Bloom IVF Centre, Merck KGaA, Ferring B.V., Hamilton Thorne, Inc., Nikon Corporation, Nidacon International AB, Laboratoire CCD, Esco Micro Pte. Ltd. |
Ans. The Compound Annual Growth Rate for Assisted Reproductive Technology Market over the forecast period is 7.68%.
Ans. The Assisted Reproductive Technology (ART) Market size was estimated at USD 28.33 billion in 2023 and is expected to reach USD 55.09 billion by 2032 with a growing CAGR of 7.68% during the forecast period of 2024-2032.
Ans. The major key players are Vitrolife AB,Merck KGaA, FUJIFILM Irvine Scientific,Ferring B.V., European Sperm Bank, Cosmos Biomedical Ltd.,Cryolab Ltd.,Bloom IVF Centre, Microm U.K. Ltd., CooperSurgical, Inc. and others.
Ans. North America is the fastest growing region of the Assisted Reproductive Technology Market.
Ans. The rising need of overcoming infertility
1. Introduction
1.1 Market Definition
1.2 Scope (Inclusion and Exclusions)
1.3 Research Assumptions
2. Executive Summary
2.1 Market Overview
2.2 Regional Synopsis
2.3 Competitive Summary
3. Research Methodology
3.1 Top-Down Approach
3.2 Bottom-up Approach
3.3. Data Validation
3.4 Primary Interviews
4. Market Dynamics Impact Analysis
4.1 Market Driving Factors Analysis
4.1.2 Drivers
4.1.2 Restraints
4.1.3 Opportunities
4.1.4 Challenges
4.2 PESTLE Analysis
4.3 Porter’s Five Forces Model
5. Statistical Insights and Trends Reporting
5.1 Incidence and Prevalence of Infertility (2023)
5.2 ART Procedure Trends (2023), by Region
5.3 Success Rates of ART (2023)
5.4 Technological Advancements in ART (2023)
5.5 Demographic and Societal Trends Impacting ART (2023)
6. Competitive Landscape
6.1 List of Major Companies, By Region
6.2 Market Share Analysis, By Region
6.3 Service Benchmarking
6.3.1 Service specifications and features
6.3.2 Pricing
6.4 Strategic Initiatives
6.4.1 Marketing and promotional activities
6.4.2 Distribution and Supply Chain Strategies
6.4.3 Expansion plans and new Service launches
6.4.4 Strategic partnerships and collaborations
6.5 Technological Advancements
6.6 Market Positioning and Branding
7. Assisted Reproductive Technology Market Segmentation, by Type
7.1 Chapter Overview
7.2 In-vitro fertilization (IVF)
7.2.1 In-vitro fertilization (IVF) Market Trends Analysis (2020-2032)
7.2.2 In-vitro fertilization (IVF) Market Size Estimates and Forecasts to 2032 (USD Billion)
7.2.3 Fresh Donor
7.2.3.1 Fresh Donor Market Trends Analysis (2020-2032)
7.2.3.2 Fresh Donor Market Size Estimates and Forecasts to 2032 (USD Billion)
7.2.4 Frozen Donor
7.2.4.1 Frozen Donor Market Trends Analysis (2020-2032)
7.2.4.2 Frozen Donor Market Size Estimates and Forecasts to 2032 (USD Billion)
7.2.5 Fresh Non-Donor
7.2.5.1 Fresh Non-Donor Market Trends Analysis (2020-2032)
7.2.5.2 Fresh Non-Donor Market Size Estimates and Forecasts to 2032 (USD Billion)
7.2.6 Frozen Non-Donor
7.2.6.1 Frozen Non-Donor Market Trends Analysis (2020-2032)
7.2.6.2 Frozen Non-Donor Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3 Artificial Insemination
7.3.1 Artificial Insemination Market Trends Analysis (2020-2032)
7.3.2 Artificial Insemination Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.3 Intrauterine Insemination
7.3.3.1 Intrauterine Insemination Market Trends Analysis (2020-2032)
7.3.3.2 Intrauterine Insemination Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.4 Intracervical Insemination
7.3.4.1 Intracervical Insemination Market Trends Analysis (2020-2032)
7.3.4.2 Intracervical Insemination Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.5 Intravaginal Insemination
7.3.5.1 Intravaginal Insemination Market Trends Analysis (2020-2032)
7.3.5.2 Intravaginal Insemination Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3.6 Intratubal Insemination
7.3.6.1 Intratubal Insemination Market Trends Analysis (2020-2032)
7.3.6.2 Intratubal Insemination Market Size Estimates and Forecasts to 2032 (USD Billion)
8. Assisted Reproductive Technology Market Segmentation, by End-use
8.2 Fertility Clinics & Other Settings
8.2.1 Fertility Clinics & Other Settings Market Trends Analysis (2020-2032)
8.2.2 Fertility Clinics & Other Settings Market Size Estimates and Forecasts to 2032 (USD Billion)
8.3 Hospitals and Others
8.3.1 Hospitals and Others Market Trends Analysis (2020-2032)
8.3.2 Hospitals and Others Market Size Estimates and Forecasts to 2032 (USD Billion)
9. Regional Analysis
9.1 Chapter Overview
9.2 North America
9.2.1 Trends Analysis
9.2.2 North America Assisted Reproductive Technology Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.2.3 North America Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.2.4 North America Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.2.5 USA
9.2.5.1 USA Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.2.5.2 USA Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.2.6 Canada
9.2.6.1 Canada Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.2.6.2 Canada Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.2.7 Mexico
9.2.7.1 Mexico Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.2.7.2 Mexico Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.3 Europe
9.3.1 Eastern Europe
9.3.1.1 Trends Analysis
9.3.1.2 Eastern Europe Assisted Reproductive Technology Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.3.1.3 Eastern Europe Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.3.1.4 Eastern Europe Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.3.1.5 Poland
9.3.1.5.1 Poland Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.3.1.5.2 Poland Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.3.1.6 Romania
9.3.1.6.1 Romania Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.3.1.6.2 Romania Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.3.1.7 Hungary
9.3.1.7.1 Hungary Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.3.1.7.2 Hungary Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.3.1.8 Turkey
9.3.1.8.1 Turkey Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.3.1.8.2 Turkey Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.3.1.9 Rest of Eastern Europe
9.3.1.9.1 Rest of Eastern Europe Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.3.1.9.2 Rest of Eastern Europe Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.3.2 Western Europe
9.3.2.1 Trends Analysis
9.3.2.2 Western Europe Assisted Reproductive Technology Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.3.2.3 Western Europe Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.3.2.4 Western Europe Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.3.2.5 Germany
9.3.2.5.1 Germany Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.3.2.5.2 Germany Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.3.2.6 France
9.3.2.6.1 France Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.3.2.6.2 France Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.3.2.7 UK
9.3.2.7.1 UK Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.3.2.7.2 UK Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.3.2.8 Italy
9.3.2.8.1 Italy Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.3.2.8.2 Italy Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.3.2.9 Spain
9.3.2.9.1 Spain Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.3.2.9.2 Spain Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.3.2.10 Netherlands
9.3.2.10.1 Netherlands Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.3.2.10.2 Netherlands Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.3.2.11 Switzerland
9.3.2.11.1 Switzerland Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.3.2.11.2 Switzerland Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.3.2.12 Austria
9.3.2.12.1 Austria Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.3.2.12.2 Austria Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.3.2.13 Rest of Western Europe
9.3.2.13.1 Rest of Western Europe Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.3.2.13.2 Rest of Western Europe Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.4 Asia Pacific
9.4.1 Trends Analysis
9.4.2 Asia Pacific Assisted Reproductive Technology Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.4.3 Asia Pacific Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.4.4 Asia Pacific Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.4.5 China
9.4.5.1 China Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.4.5.2 China Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.4.6 India
9.4.5.1 India Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.4.5.2 India Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.4.5 Japan
9.4.5.1 Japan Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.4.5.2 Japan Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.4.6 South Korea
9.4.6.1 South Korea Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.4.6.2 South Korea Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.4.7 Vietnam
9.4.7.1 Vietnam Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.2.7.2 Vietnam Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.4.8 Singapore
9.4.8.1 Singapore Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.4.8.2 Singapore Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.4.9 Australia
9.4.9.1 Australia Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.4.9.2 Australia Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.4.10 Rest of Asia Pacific
9.4.10.1 Rest of Asia Pacific Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.4.10.2 Rest of Asia Pacific Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.5 Middle East and Africa
9.5.1 Middle East
9.5.1.1 Trends Analysis
9.5.1.2 Middle East Assisted Reproductive Technology Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.5.1.3 Middle East Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.5.1.4 Middle East Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.5.1.5 UAE
9.5.1.5.1 UAE Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.5.1.5.2 UAE Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.5.1.6 Egypt
9.5.1.6.1 Egypt Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.5.1.6.2 Egypt Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.5.1.7 Saudi Arabia
9.5.1.7.1 Saudi Arabia Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.5.1.7.2 Saudi Arabia Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.5.1.8 Qatar
9.5.1.8.1 Qatar Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.5.1.8.2 Qatar Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.5.1.9 Rest of Middle East
9.5.1.9.1 Rest of Middle East Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.5.1.9.2 Rest of Middle East Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.5.2 Africa
9.5.2.1 Trends Analysis
9.5.2.2 Africa Assisted Reproductive Technology Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.5.2.3 Africa Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.5.2.4 Africa Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.5.2.5 South Africa
9.5.2.5.1 South Africa Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.5.2.5.2 South Africa Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.5.2.6 Nigeria
9.5.2.6.1 Nigeria Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.5.2.6.2 Nigeria Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.6 Latin America
9.6.1 Trends Analysis
9.6.2 Latin America Assisted Reproductive Technology Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.6.3 Latin America Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.6.4 Latin America Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.6.5 Brazil
9.6.5.1 Brazil Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.6.5.2 Brazil Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.6.6 Argentina
9.6.6.1 Argentina Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.6.6.2 Argentina Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.6.7 Colombia
9.6.7.1 Colombia Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.6.7.2 Colombia Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
9.6.8 Rest of Latin America
9.6.8.1 Rest of Latin America Assisted Reproductive Technology Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
9.6.8.2 Rest of Latin America Assisted Reproductive Technology Market Estimates and Forecasts, by End-use (2020-2032) (USD Billion)
10. Company Profiles
10.1 Cosmos Biomedical Ltd.
10.1.1 Company Overview
10.1.2 Financial
10.1.3 Products/ Services Offered
10.1.4 SWOT Analysis
10.2 Microm U.K. Ltd.
10.2.1 Company Overview
10.2.2 Financial
10.2.3 Products/ Services Offered
10.2.4 SWOT Analysis
10.3 CooperSurgical, Inc.
10.3.1 Company Overview
10.3.2 Financial
10.3.3 Products/ Services Offered
10.3.4 SWOT Analysis
10.4 FUJIFILM Irvine Scientific
10.4.1 Company Overview
10.4.2 Financial
10.4.3 Products/ Services Offered
10.4.4 SWOT Analysis
10.5 Cryolab Ltd.
10.5.1 Company Overview
10.5.2 Financial
10.5.3 Products/ Services Offered
10.5.4 SWOT Analysis
10.6 European Sperm Bank
10.6.1 Company Overview
10.6.2 Financial
10.6.3 Products/ Services Offered
10.6.4 SWOT Analysis
10.7 Bloom IVF Centre
10.7.1 Company Overview
10.7.2 Financial
10.7.3 Products/ Services Offered
10.7.4 SWOT Analysis
10.8 Hamilton Thorne, Inc.
10.8.1 Company Overview
10.8.2 Financial
10.8.3 Products/ Services Offered
10.8.4 SWOT Analysis
10.9 Esco Micro Pte. Ltd.
10.9.1 Company Overview
10.9.2 Financial
10.9.3 Products/ Services Offered
10.9.4 SWOT Analysis
10.10 Nikon Corporation
10.10.1 Company Overview
10.10.2 Financial
10.10.3 Products/ Services Offered
10.10.4 SWOT Analysis
11. Use Cases and Best Practices
12. Conclusion
An accurate research report requires proper strategizing as well as implementation. There are multiple factors involved in the completion of good and accurate research report and selecting the best methodology to compete the research is the toughest part. Since the research reports we provide play a crucial role in any company’s decision-making process, therefore we at SNS Insider always believe that we should choose the best method which gives us results closer to reality. This allows us to reach at a stage wherein we can provide our clients best and accurate investment to output ratio.
Each report that we prepare takes a timeframe of 350-400 business hours for production. Starting from the selection of titles through a couple of in-depth brain storming session to the final QC process before uploading our titles on our website we dedicate around 350 working hours. The titles are selected based on their current market cap and the foreseen CAGR and growth.
The 5 steps process:
Step 1: Secondary Research:
Secondary Research or Desk Research is as the name suggests is a research process wherein, we collect data through the readily available information. In this process we use various paid and unpaid databases which our team has access to and gather data through the same. This includes examining of listed companies’ annual reports, Journals, SEC filling etc. Apart from this our team has access to various associations across the globe across different industries. Lastly, we have exchange relationships with various university as well as individual libraries.

Step 2: Primary Research
When we talk about primary research, it is a type of study in which the researchers collect relevant data samples directly, rather than relying on previously collected data. This type of research is focused on gaining content specific facts that can be sued to solve specific problems. Since the collected data is fresh and first hand therefore it makes the study more accurate and genuine.
We at SNS Insider have divided Primary Research into 2 parts.
Part 1 wherein we interview the KOLs of major players as well as the upcoming ones across various geographic regions. This allows us to have their view over the market scenario and acts as an important tool to come closer to the accurate market numbers. As many as 45 paid and unpaid primary interviews are taken from both the demand and supply side of the industry to make sure we land at an accurate judgement and analysis of the market.
This step involves the triangulation of data wherein our team analyses the interview transcripts, online survey responses and observation of on filed participants. The below mentioned chart should give a better understanding of the part 1 of the primary interview.

Part 2: In this part of primary research the data collected via secondary research and the part 1 of the primary research is validated with the interviews from individual consultants and subject matter experts.
Consultants are those set of people who have at least 12 years of experience and expertise within the industry whereas Subject Matter Experts are those with at least 15 years of experience behind their back within the same space. The data with the help of two main processes i.e., FGDs (Focused Group Discussions) and IDs (Individual Discussions). This gives us a 3rd party nonbiased primary view of the market scenario making it a more dependable one while collation of the data pointers.
Step 3: Data Bank Validation
Once all the information is collected via primary and secondary sources, we run that information for data validation. At our intelligence centre our research heads track a lot of information related to the market which includes the quarterly reports, the daily stock prices, and other relevant information. Our data bank server gets updated every fortnight and that is how the information which we collected using our primary and secondary information is revalidated in real time.

Step 4: QA/QC Process
After all the data collection and validation our team does a final level of quality check and quality assurance to get rid of any unwanted or undesired mistakes. This might include but not limited to getting rid of the any typos, duplication of numbers or missing of any important information. The people involved in this process include technical content writers, research heads and graphics people. Once this process is completed the title gets uploader on our platform for our clients to read it.
Step 5: Final QC/QA Process:
This is the last process and comes when the client has ordered the study. In this process a final QA/QC is done before the study is emailed to the client. Since we believe in giving our clients a good experience of our research studies, therefore, to make sure that we do not lack at our end in any way humanly possible we do a final round of quality check and then dispatch the study to the client.
By Type
In-vitro fertilization (IVF)
Fresh Donor
Frozen Donor
Fresh Non-Donor
Frozen Non-Donor
Artificial Insemination
Intrauterine Insemination
Intracervical Insemination
Intravaginal Insemination
Intratubal Insemination
By End-use
Fertility Clinics & other settings
Hospitals And Others
Request for Segment Customization as per your Business Requirement: Segment Customization Request
Regional Coverage:
North America
US
Canada
Mexico
Europe
Eastern Europe
Poland
Romania
Hungary
Turkey
Rest of Eastern Europe
Western Europe
Germany
France
UK
Italy
Spain
Netherlands
Switzerland
Austria
Rest of Western Europe
Asia Pacific
China
India
Japan
South Korea
Vietnam
Singapore
Australia
Rest of Asia Pacific
Middle East & Africa
Middle East
UAE
Egypt
Saudi Arabia
Qatar
Rest of Middle East
Africa
Nigeria
South Africa
Rest of Africa
Latin America
Brazil
Argentina
Colombia
Rest of Latin America
Request for Country Level Research Report: Country Level Customization Request
Available Customization
With the given market data, SNS Insider offers customization as per the company’s specific needs. The following customization options are available for the report:
Detailed Volume Analysis
Criss-Cross segment analysis (e.g. Product X Application)
Competitive Product Benchmarking
Geographic Analysis
Additional countries in any of the regions
Customized Data Representation
Detailed analysis and profiling of additional market players
The Neuroprosthetics Market size was valued at USD 12.69 billion in 2023 and is expected to reach USD 38.93 billion by 2032, growing at a CAGR of 13.28%.
The Clinical Trial Equipment & Ancillary Solutions Market Size was valued at USD 2.23 billion in 2023 and is expected to reach USD 4.39 billion by 2032 and grow at a CAGR of 7.84% over the forecast period 2024-2032.
The Blood Pressure Monitors Market size was valued at USD 1.81 Billion in 2023 and is estimated to grow to USD 4.28 Billion by 2032, with a 10.04% CAGR.
The Process Analytical Technology Market Size was valued at USD 3.50 Billion in 2023, and is expected to reach USD 10.19 Billion by 2032, and grow at a CAGR of 13.29%.
The Analytical Instrumentation Market Size was valued at USD 53 Billion in 2023 and is expected to reach USD 84.1 billion by 2032, growing at a CAGR of 5.3% by 2024-2032.
Anesthesia Monitoring Devices Market was valued at USD 2.03 Bn in 2023 and is expected to reach USD 5.26 Bn by 2032, growing at a CAGR of 11.21% from 2024-2032.
Hi! Click one of our member below to chat on Phone