Antisense and RNAi Therapeutics Market Scope:
Get More Information on Antisense and RNAi Therapeutics Market - Request Sample Report
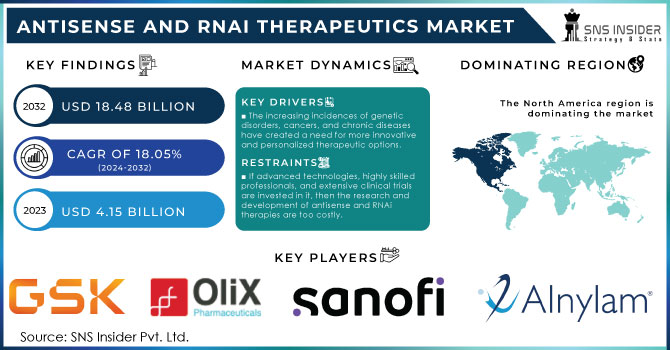
The Antisense and RNAi Therapeutics Market size is projected to reach USD 18.48 billion by 2032, growing at a CAGR of 18.05% over 2024-2032. The market was valued at USD 4.15 billion in 2023.
The key driving factors for the demand of Antisense and RNAi therapeutics include genetic disorders and chronic diseases, among which the incidence is continuously growing. It is said that around 10% of the population in the U.S. suffers from rare diseases, most of which involve some kind of genetic component; hence, antisense and RNAi are important ways of treatment. According to the NIH, over 7,000 rare diseases affect roughly 30 million Americans, giving a sizeable patient population to these new therapies. Similarly, government initiatives like the Precision Medicine Initiative have also driven demand as a result of a renewed focus on personalized medicine. The Precision Medicine Initiative is one aimed at treating medical conditions based on an individual's specific genetic makeup; antisense and RNAi technologies are at the forefront. Other significant drivers include investment in biotechnology research; for example, the US government increased funding by 7% in 2023 to advance the development of new therapies. In addition, due to the FDA's fast-track approval process for breakthrough therapies, antisense and RNAi drugs have reached the market much quicker, with 40% of new approvals in 2023 being from this category. These factors cumulatively mark an increasing role of antisense and RNAi therapeutics in unmet medical needs in the U.S.
Key trends include rapid adoption rates for RNA interference technologies, which have so far usually shown promise in targeting and silencing certain disease-causing genes. According to government data, the rate of adoption of RNAi-based therapies surged by approximately 35% annually over the last three years, reflecting growing confidence in these treatments' efficacy and safety. Further, the demand for antisense oligonucleotides is on the rise because it has now become possible to regulate gene activity with their help very effectively and precisely. Clinical trials of therapies based on ASO are growing 27% compared to the period between 2020 and 2023. Strong government support of biotech innovation also drives the segment: federal funding for research into RNAi increased 22% in the last fiscal year. Thus, FDA expedited approval of antisense and RNAi drugs reduces time-to-market by 15%, a factor that should encourage more biotech firms to invest in these technologies.
Market Dynamics:
Drivers:
The increasing incidences of genetic disorders, cancers, and chronic diseases have created a need for more innovative and personalized therapeutic options. Therefore, antisense and RNAi therapeutics marketplace increased.
The high investments by pharmaceutical companies, governments, and research institutions in R&D accelerate the process of discovery and development of new antisense and RNAi therapies, hence expanding product pipelines.
Innovations like drug delivery systems with lipid nanoparticles and conjugation technologies have enhanced antisense and RNAi stability, specificity, and efficacy, thus making them more viable and effective for human application.
The Antisense and RNAi Therapeutics Market has been significantly developed by innovations in the drug delivery system, such as the development of lipid nanoparticles and conjugation technologies. Such innovations have enhanced their stability, specificity, and efficacy, thus making them more viable and effective in clinical settings. According to government health agencies, the introduction of lipid nanoparticles in RNAi therapeutics increased drug delivery efficiencies by approximately 45% on average, which allows diseases of specific cells to be targeted with minimal off-target effects. Conjugation technologies have also been employed to enhance therapeutic indices by approximately 30%, thus allowing for the use of lower dosages with greatly increased potencies and diminished toxicities.
This has led to a manifold increase in the clinical success rate of RNA-based therapies, with government reports quoting a 20% increase in approval rates of antisense and RNAi drugs in the last five years. In addition, the therapeutic uses of RNA-based drugs have accelerated the pace of development and positioned them as a game-changing approach to treat genetic and rare diseases. Owing to this, the Antisense and RNAi Therapeutics Market will see very strong growth as these technologies improve further. For that, increased clinical adoption and effectiveness will prove to be major drivers.
Restrains:
If advanced technologies, highly skilled professionals, and extensive clinical trials are invested in it, then the research and development of antisense and RNAi therapies are too costly. All this can be very discouraging for companies-even for smaller biotech organizations-due to extravagance in prices.
Manufacturing antisense and RNAi therapeutics involves very complex processes that require advanced and sophisticated technologies. Generally, scaling up production or manufacturing operations results in inconsistent batch-to-batch quality.
Manufacturing antisense and RNAi therapeutics are complex, with highly specialized processes that pose substantial challenges in scalability into commercial production. These challenges emanate from the precision with which the synthesis of oligonucleotides-the active ingredients in antisense and RNAi therapies-must be controlled; such control is demanding both in technique and in quality controls. Ensuring batch-to-batch consistency is critical, as even slight variations may affect therapeutic efficacy and safety. Data from the U.S. Food and Drug Administration estimates that approximately 18% of manufacturing deviations reported for the biopharmaceutical industry address problems related to the manufacturing process of nucleic acid-based therapies, including antisense and RNAi.
Furthermore, the EMA comments that about 22% of applications for RNA-based therapies face delays due to manufacturing complexities, underlining the challenges faced by manufacturers in this growing market. On account of the rising demand due to their potential to treat a range of genetic disorders and cancers, heavy investments are being made by the industry in improving production technologies and processes to enhance scalability and ensure consistent quality to allow successful commercialization.
Market Segmentation Analysis:
By Technology
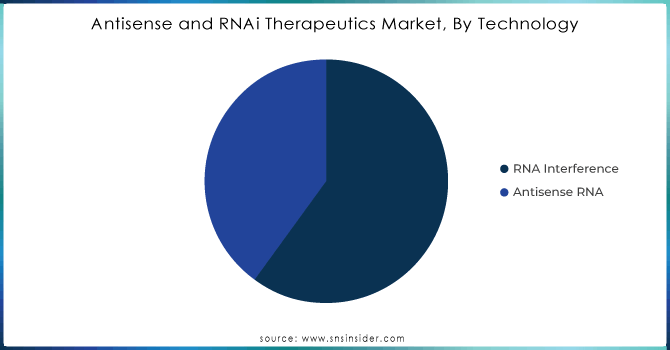
RNAi technology captured the maximum share in 2023, with close to 60% of the total revenue. The main contribution has come from RNAi, which effectively silences specific gene expressions and is considered important in finding its application to treat various genetic disorders and cancers. Further, the strong clinical pipeline and rising regulatory approvals for RNAi therapeutics accelerate the wide adoption of this therapeutic modality; it is expected to grow at a CAGR of 22% from 2024 to 2032. Antisense RNA technology, on the other hand, held around 40% share of the market in 2023 and promises high growth due to its novelty in the mechanism of action of suppressing messenger RNA (mRNA) for ablation in the production of disease-causing proteins. Antisense therapeutics in RNA have gained momentum and adaptation, particularly in the treatment of neurological disorders and rare diseases. This segment is expected to grow at a CAGR of 19% in the same forecast period. Both technologies are growing efforts toward broadening the landscape in RNA-based therapeutics to respond to unmet medical needs.
Need any customization research on Antisense and RNAi Therapeutics Market - Enquiry Now
By Route of Administration
The intravenous route of injection leads the market share owing to its rapid and active delivery of therapeutics into the blood circulation. It held a share of about 45% in 2023, resulting from its wide usage in clinical settings for treating a number of genetic disorders and cancers. Intrathecal injections, as represented by medication administration directly into cerebrospinal fluid, made up about 25% of the market, largely utilized for neurological disorders such as spinal muscular atrophy and amyotrophic lateral sclerosis. Subcutaneous injections accounted for a share of about 20%, with an ease of administration that, therefore, ensues a potentiality for self-administration in chronic conditions such as diabetes and certain types of cancers. While the remaining 10% was contributed to other delivery methods, which include oral, topical, and inhalation routes, and are gaining popularity because of continuous development in the fields of drug formulation and delivery technologies.
Key Players:
The major players are GSK plc, Olix Pharmaceuticals, Inc., Sanofi, Alnylam Pharmaceuticals, Inc., Arbutus Biopharma, Benitec Biopharma Inc., Silence Therapeutics, Ionis Pharmaceuticals, Inc, Sarepta Therapeutics, Percheron Therapeutics Limited and other players.
Regional Analysis:
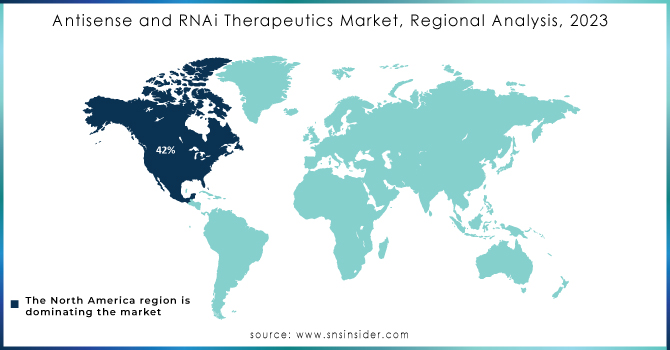
The North American antisense and RNAi therapeutics market is anticipated to witness high growth over the forecast period. Other factors include an increasing prevalence of chronic diseases such as cancer and neurological disorders. Its vast share of the global market-owning about 42% of the total revenue in 2023-underlines dominance in the region. A growth of this nature has several R&D activities, with significant investments by both private and public sectors. For instance, the United States alone contributes to over 70% in North America's market share. Regulatory support by agencies like the FDA has further accelerated the approval of numerous RNA-based therapies, thereby continuing to drive the market growth. The adoption of advanced technologies and the presence of key pharmaceutical companies further establish the region's competitive advantage. Because of the rising demand for targeted therapies with minimal side effects, the North American market is likely to achieve 15.5% CAGR by 2032. It also forms a significant contribution, as its healthcare segment increasingly working toward RNAi-based treatments adds to overall regional growth. The continued emphasis on personalized medicine and the gene-silencing technologies secures the position of North America in the global antisense and RNAi therapeutics market.
Recent Developments:
Ionis Pharmaceuticals: Ionis Pharmaceuticals announced positive Phase 3 trial results with its antisense drug, ION363, designed to treat amyotrophic lateral sclerosis caused by mutations in the fused in sarcoma gene. It showed that the drug significantly reduces disease progression and may be on the right track toward FDA approval.
Alnylam Pharmaceuticals: The Company's RNAi therapeutic, Oxlumo®, also referred to as lumasiran, was approved by the FDA for the treatment of primary hyperoxaluria type 1 among pediatric and adult patients. This is considered a landmark approval since it is the first-ever RNAi therapy approved for this rare genetic disorder.
| Report Attributes | Details |
| Market Size in 2023 | USD 4.15 billion |
| Market Size by 2032 | USD 18.48 Billion |
| CAGR | CAGR of 18.05% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Technology: (RNA Interference, Antisense RNA) • By Application: (Genetic Disease, Cancer, Infectious Disease, Neurodegenerative Disorders, Cardiometabolic & Renal Disorders, Ocular Disorders, Respiratory Disorders, Skin Disorders) • By Route of Administration: (Intravenous Injections, Intrathecal Injections, Subcutaneous Injections, Other Delivery Methods) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | GSK plc, Olix Pharmaceuticals, Inc., Sanofi, Alnylam Pharmaceuticals, Inc., Arbutus Biopharma, Benitec Biopharma Inc., Silence Therapeutics, Ionis Pharmaceuticals, Inc, Sarepta Therapeutics, Percheron Therapeutics Limited |
| Key Drivers | Innovations like drug delivery systems with lipid nanoparticles and conjugation technologies, have enhanced antisense and RNAi stability, specificity, and efficacy, thus making them more viable and effective for human application. |
| RESTRAINTS | Manufacturing antisense and RNAi therapeutics involves very complex processes that require advanced and sophisticated technologies |
Ans: The Antisense and RNAi therapeutics Market size is projected to reach USD 18.48 billion by 2032.
Ans: The Antisense and RNAi therapeutics Market will be growing at a CAGR of 18.05% over 2024-2032.
Ans: If advanced technologies, highly skilled professionals, and extensive clinical trials are invested in it, then the research and development of antisense and RNAi therapies are too costly.
Ans: The high investments by pharmaceutical companies, governments, and research institutions in R&D accelerate the process of discovery and development of new antisense and RNAi therapies, hence expanding product pipelines.
Ans: North America will be dominating the Antisense and RNAi therapeutics Market over the forecast period.
Table of Contents
1. Introduction
1.1 Market Definition
1.2 Scope (Inclusion and Exclusions)
1.3 Research Assumptions
2. Executive Summary
2.1 Market Overview
2.2 Regional Synopsis
2.3 Competitive Summary
3. Research Methodology
3.1 Top-Down Approach
3.2 Bottom-up Approach
3.3. Data Validation
3.4 Primary Interviews
4. Market Dynamics Impact Analysis
4.1 Market Driving Factors Analysis
4.1.1 Drivers
4.1.2 Restraints
4.1.3 Opportunities
4.1.4 Challenges
4.2 PESTLE Analysis
4.3 Porter’s Five Forces Model
5. Statistical Insights and Trends Reporting
5.1 Incidence and Prevalence (2023)
5.2 Prescription Trends, (2023), by Region
5.3 Device Volume, by Region (2020-2032)
5.4 Healthcare Spending, by Region, (Government, Commercial, Private, Out-of-Pocket), 2023
6. Competitive Landscape
6.1 List of Major Companies, By Region
6.2 Market Share Analysis, By Region
6.3 Product Benchmarking
6.3.1 Product specifications and features
6.3.2 Pricing
6.4 Strategic Initiatives
6.4.1 Marketing and promotional activities
6.4.2 Distribution and supply chain strategies
6.4.3 Expansion plans and new product launches
6.4.4 Strategic partnerships and collaborations
6.5 Technological Advancements
6.6 Market Positioning and Branding
7. Antisense and RNAi therapeutics Market Segmentation, by Technology
7.1 Chapter Overview
7.2 RNA Interference
7.2.1 RNA Interference Market Trends Analysis (2020-2032)
7.2.2 RNA Interference Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3 Antisense RNA
7.3.1 Antisense RNA Market Trends Analysis (2020-2032)
7.3.2 Antisense RNA Market Size Estimates and Forecasts to 2032 (USD Billion)
8. Antisense and RNAi therapeutics Market Segmentation, by Application
8.1 Chapter Overview
8.2 Genetic Disease
8.2.1 Genetic Disease Market Trends Analysis (2020-2032)
8.2.2 Genetic Disease Market Size Estimates and Forecasts to 2032 (USD Billion)
8.3 Cancer
8.3.1 Cancer Market Trends Analysis (2020-2032)
8.3.2 Cancer Market Size Estimates and Forecasts to 2032 (USD Billion)
8.4 Infectious Diseases
8.4.1 Infectious Disease Market Trends Analysis (2020-2032)
8.4.2 Infectious Disease Market Size Estimates and Forecasts to 2032 (USD Billion)
8.5 Neurodegenerative Disorders
8.5.1 Neurodegenerative Disorders Market Trends Analysis (2020-2032)
8.5.2 Neurodegenerative Disorders Market Size Estimates and Forecasts to 2032 (USD Billion)
8.6 Cardiometabolic & Renal Disorders
8.6.1 Cardiometabolic & Renal Disorders Market Trends Analysis (2020-2032)
8.6.2 Cardiometabolic & Renal Disorders Market Size Estimates and Forecasts to 2032 (USD Billion)
8.7 Ocular Disorders
8.7.1 Ocular Disorders Market Trends Analysis (2020-2032)
8.7.2 Ocular Disorders Market Size Estimates and Forecasts to 2032 (USD Billion)
8.8 Respiratory Disorders
8.8.1 Respiratory Disorders Market Trends Analysis (2020-2032)
8.8.2 Respiratory Disorders Market Size Estimates and Forecasts to 2032 (USD Billion)
8.9 Skin Disorders
8.9.1 Skin Disorders Market Trends Analysis (2020-2032)
8.9.2 Skin Disorders Market Size Estimates and Forecasts to 2032 (USD Billion)
9. Antisense and RNAi therapeutics Market Segmentation, by Route of Administration
9.1 Chapter Overview
9.2 Intravenous Injections
9.2.1 Intravenous Injections Market Trends Analysis (2020-2032)
9.2.2 Intravenous Injections Market Size Estimates and Forecasts to 2032 (USD Billion)
9.3 Intrathecal Injections
9.3.1 Intrathecal Injections Market Trends Analysis (2020-2032)
9.3.2 Intrathecal Injections Market Size Estimates and Forecasts to 2032 (USD Billion)
9.3 Subcutaneous Injections
9.3.1 Subcutaneous Injections Market Trends Analysis (2020-2032)
9.3.2 Subcutaneous Injections Market Size Estimates and Forecasts to 2032 (USD Billion)
9.4 Other Delivery Methods
9.4.1 Other Delivery Methods Market Trends Analysis (2020-2032)
9.4.2 Other Delivery Methods Market Size Estimates and Forecasts to 2032 (USD Billion)
10. Regional Analysis
10.1 Chapter Overview
10.2 North America
10.2.1 Trends Analysis
10.2.2 North America Antisense and RNAi therapeutics Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.2.3 North America Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.2.4 North America Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.2.5 North America Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.2.6 USA
10.2.6.1 USA Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.2.6.2 USA Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.2.6.3 USA Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.2.7 Canada
10.2.7.1 Canada Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.2.7.2 Canada Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.2.7.3 Canada Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.2.8 Mexico
10.2.8.1 Mexico Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.2.8.2 Mexico Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.2.8.3 Mexico Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.3 Europe
10.3.1 Eastern Europe
10.3.1.1 Trends Analysis
10.3.1.2 Eastern Europe Antisense and RNAi therapeutics Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.3.1.3 Eastern Europe Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.3.1.4 Eastern Europe Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.3.1.5 Eastern Europe Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.3.1.6 Poland
10.3.1.6.1 Poland Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.3.1.6.2 Poland Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.3.1.6.3 Poland Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.3.1.7 Romania
10.3.1.7.1 Romania Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.3.1.7.2 Romania Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.3.1.7.3 Romania Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.3.1.8 Hungary
10.3.1.8.1 Hungary Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.3.1.8.2 Hungary Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.3.1.8.3 Hungary Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.3.1.9 Turkey
10.3.1.9.1 Turkey Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.3.1.9.2 Turkey Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.3.1.9.3 Turkey Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.3.1.10 Rest of Eastern Europe
10.3.1.10.1 Rest of Eastern Europe Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.3.1.10.2 Rest of Eastern Europe Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.3.1.10.3 Rest of Eastern Europe Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.3.2 Western Europe
10.3.2.1 Trends Analysis
10.3.2.2 Western Europe Antisense and RNAi therapeutics Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.3.2.3 Western Europe Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.3.2.4 Western Europe Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.3.2.5 Western Europe Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.3.2.6 Germany
10.3.2.6.1 Germany Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.3.2.6.2 Germany Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.3.2.6.3 Germany Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.3.2.7 France
10.3.2.7.1 France Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.3.2.7.2 France Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.3.2.7.3 France Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.3.2.8 UK
10.3.2.8.1 UK Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.3.2.8.2 UK Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.3.2.8.3 UK Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.3.2.9 Italy
10.3.2.9.1 Italy Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.3.2.9.2 Italy Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.3.2.9.3 Italy Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.3.2.10 Spain
10.3.2.10.1 Spain Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.3.2.10.2 Spain Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.3.2.10.3 Spain Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.3.2.11 Netherlands
10.3.2.11.1 Netherlands Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.3.2.11.2 Netherlands Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.3.2.11.3 Netherlands Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.3.2.12 Switzerland
10.3.2.12.1 Switzerland Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.3.2.12.2 Switzerland Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.3.2.12.3 Switzerland Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.3.2.13 Austria
10.3.2.13.1 Austria Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.3.2.13.2 Austria Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.3.2.13.3 Austria Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.3.2.14 Rest of Western Europe
10.3.2.14.1 Rest of Western Europe Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.3.2.14.2 Rest of Western Europe Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.3.2.14.3 Rest of Western Europe Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.4 Asia Pacific
10.4.1 Trends Analysis
10.4.2 Asia Pacific Antisense and RNAi therapeutics Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.4.3 Asia Pacific Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.4.4 Asia Pacific Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.4.5 Asia Pacific Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.4.6 China
10.4.6.1 China Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.4.6.2 China Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.4.6.3 China Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.4.7 India
10.4.7.1 India Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.4.7.2 India Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.4.7.3 India Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.4.8 Japan
10.4.8.1 Japan Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.4.8.2 Japan Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.4.8.3 Japan Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.4.9 South Korea
10.4.9.1 South Korea Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.4.9.2 South Korea Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.4.9.3 South Korea Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.4.10 Vietnam
10.4.10.1 Vietnam Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.4.10.2 Vietnam Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.4.10.3 Vietnam Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.4.11 Singapore
10.4.11.1 Singapore Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.4.11.2 Singapore Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.4.11.3 Singapore Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.4.12 Australia
10.4.12.1 Australia Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.4.12.2 Australia Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.4.12.3 Australia Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.4.13 Rest of Asia Pacific
10.4.13.1 Rest of Asia Pacific Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.4.13.2 Rest of Asia Pacific Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.4.13.3 Rest of Asia Pacific Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.5 Middle East and Africa
10.5.1 Middle East
10.5.1.1 Trends Analysis
10.5.1.2 Middle East Antisense and RNAi therapeutics Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.5.1.3 Middle East Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.5.1.4 Middle East Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.5.1.5 Middle East Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.5.1.6 UAE
10.5.1.6.1 UAE Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.5.1.6.2 UAE Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.5.1.6.3 UAE Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.5.1.7 Egypt
10.5.1.7.1 Egypt Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.5.1.7.2 Egypt Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.5.1.7.3 Egypt Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.5.1.8 Saudi Arabia
10.5.1.8.1 Saudi Arabia Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.5.1.8.2 Saudi Arabia Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.5.1.8.3 Saudi Arabia Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.5.1.9 Qatar
10.5.1.9.1 Qatar Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.5.1.9.2 Qatar Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.5.1.9.3 Qatar Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.5.1.10 Rest of Middle East
10.5.1.10.1 Rest of Middle East Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.5.1.10.2 Rest of Middle East Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.5.1.10.3 Rest of Middle East Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.5.2 Africa
10.5.2.1 Trends Analysis
10.5.2.2 Africa Antisense and RNAi therapeutics Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.5.2.3 Africa Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.5.2.4 Africa Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.5.2.5 Africa Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.5.2.6 South Africa
10.5.2.6.1 South Africa Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.5.2.6.2 South Africa Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.5.2.6.3 South Africa Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.5.2.7 Nigeria
10.5.2.7.1 Nigeria Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.5.2.7.2 Nigeria Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.5.2.7.3 Nigeria Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.5.2.8 Rest of Africa
10.5.2.8.1 Rest of Africa Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.5.2.8.2 Rest of Africa Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.5.2.8.3 Rest of Africa Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.6 Latin America
10.6.1 Trends Analysis
10.6.2 Latin America Antisense and RNAi therapeutics Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.6.3 Latin America Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.6.4 Latin America Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.6.5 Latin America Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.6.6 Brazil
10.6.6.1 Brazil Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.6.6.2 Brazil Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.6.6.3 Brazil Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.6.7 Argentina
10.6.7.1 Argentina Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.6.7.2 Argentina Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.6.7.3 Argentina Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.6.8 Colombia
10.6.8.1 Colombia Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.6.8.2 Colombia Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.6.8.3 Colombia Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
10.6.9 Rest of Latin America
10.6.9.1 Rest of Latin America Antisense and RNAi therapeutics Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.6.9.2 Rest of Latin America Antisense and RNAi therapeutics Market Estimates and Forecasts, by Application (2020-2032) (USD Billion)
10.6.9.3 Rest of Latin America Antisense and RNAi therapeutics Market Estimates and Forecasts, by Route of Administration (2020-2032) (USD Billion)
11. Company Profiles
11.1 GSK plc
11.1.1 Company Overview
11.1.2 Financial
11.1.3 Products/ Services Offered
11.1.4 SWOT Analysis
11.2 Olix Pharmaceuticals Inc.
11.2.1 Company Overview
11.2.2 Financial
11.2.3 Products/ Services Offered
11.2.4 SWOT Analysis
11.3 Sanofi
11.3.1 Company Overview
11.3.2 Financial
11.3.3 Products/ Services Offered
11.3.4 SWOT Analysis
11.4 Alnylam Pharmaceuticals, Inc.
11.4.1 Company Overview
11.4.2 Financial
11.4.3 Products/ Services Offered
11.4.4 SWOT Analysis
11.5 Arbutus Biopharma
11.5.1 Company Overview
11.5.2 Financial
11.5.3 Products/ Services Offered
11.5.4 SWOT Analysis
11.6 Benitec Biopharma Inc.
11.6.1 Company Overview
11.6.2 Financial
11.6.3 Products/ Services Offered
11.6.4 SWOT Analysis
11.7 Silence Therapeutics
11.7.1 Company Overview
11.7.2 Financial
11.7.3 Products/ Services Offered
11.7.4 SWOT Analysis
11.8 Ionis Pharmaceuticals, Inc
11.8.1 Company Overview
11.8.2 Financial
11.8.3 Products/ Services Offered
11.8.4 SWOT Analysis
11.9 Sarepta Therapeutics
11.9.1 Company Overview
11.9.2 Financial
11.9.3 Products/ Services Offered
11.9.4 SWOT Analysis
11.10 Others
11.10.1 Company Overview
11.10.2 Financial
11.10.3 Products/ Services Offered
11.10.4 SWOT Analysis
12. Use Cases and Best Practices
13. Conclusion
An accurate research report requires proper strategizing as well as implementation. There are multiple factors involved in the completion of good and accurate research report and selecting the best methodology to compete the research is the toughest part. Since the research reports we provide play a crucial role in any company’s decision-making process, therefore we at SNS Insider always believe that we should choose the best method which gives us results closer to reality. This allows us to reach at a stage wherein we can provide our clients best and accurate investment to output ratio.
Each report that we prepare takes a timeframe of 350-400 business hours for production. Starting from the selection of titles through a couple of in-depth brain storming session to the final QC process before uploading our titles on our website we dedicate around 350 working hours. The titles are selected based on their current market cap and the foreseen CAGR and growth.
The 5 steps process:
Step 1: Secondary Research:
Secondary Research or Desk Research is as the name suggests is a research process wherein, we collect data through the readily available information. In this process we use various paid and unpaid databases which our team has access to and gather data through the same. This includes examining of listed companies’ annual reports, Journals, SEC filling etc. Apart from this our team has access to various associations across the globe across different industries. Lastly, we have exchange relationships with various university as well as individual libraries.

Step 2: Primary Research
When we talk about primary research, it is a type of study in which the researchers collect relevant data samples directly, rather than relying on previously collected data. This type of research is focused on gaining content specific facts that can be sued to solve specific problems. Since the collected data is fresh and first hand therefore it makes the study more accurate and genuine.
We at SNS Insider have divided Primary Research into 2 parts.
Part 1 wherein we interview the KOLs of major players as well as the upcoming ones across various geographic regions. This allows us to have their view over the market scenario and acts as an important tool to come closer to the accurate market numbers. As many as 45 paid and unpaid primary interviews are taken from both the demand and supply side of the industry to make sure we land at an accurate judgement and analysis of the market.
This step involves the triangulation of data wherein our team analyses the interview transcripts, online survey responses and observation of on filed participants. The below mentioned chart should give a better understanding of the part 1 of the primary interview.

Part 2: In this part of primary research the data collected via secondary research and the part 1 of the primary research is validated with the interviews from individual consultants and subject matter experts.
Consultants are those set of people who have at least 12 years of experience and expertise within the industry whereas Subject Matter Experts are those with at least 15 years of experience behind their back within the same space. The data with the help of two main processes i.e., FGDs (Focused Group Discussions) and IDs (Individual Discussions). This gives us a 3rd party nonbiased primary view of the market scenario making it a more dependable one while collation of the data pointers.
Step 3: Data Bank Validation
Once all the information is collected via primary and secondary sources, we run that information for data validation. At our intelligence centre our research heads track a lot of information related to the market which includes the quarterly reports, the daily stock prices, and other relevant information. Our data bank server gets updated every fortnight and that is how the information which we collected using our primary and secondary information is revalidated in real time.

Step 4: QA/QC Process
After all the data collection and validation our team does a final level of quality check and quality assurance to get rid of any unwanted or undesired mistakes. This might include but not limited to getting rid of the any typos, duplication of numbers or missing of any important information. The people involved in this process include technical content writers, research heads and graphics people. Once this process is completed the title gets uploader on our platform for our clients to read it.
Step 5: Final QC/QA Process:
This is the last process and comes when the client has ordered the study. In this process a final QA/QC is done before the study is emailed to the client. Since we believe in giving our clients a good experience of our research studies, therefore, to make sure that we do not lack at our end in any way humanly possible we do a final round of quality check and then dispatch the study to the client.
Key Segments
By Technology
RNA Interference
Antisense RNA
By Application
Genetic Disease
Cancer
Infectious Disease
Neurodegenerative Disorders
Cardiometabolic & Renal Disorders
Ocular Disorders
Respiratory Disorders
Skin Disorders
By Route of Administration
Intravenous Injections
Intrathecal Injections
Subcutaneous Injections
Other Delivery Methods
Request for Segment Customization as per your Business Requirement: Segment Customization Request
Regional Coverage:
North America
US
Canada
Mexico
Europe
Eastern Europe
Poland
Romania
Hungary
Turkey
Rest of Eastern Europe
Western Europe
Germany
France
UK
Italy
Spain
Netherlands
Switzerland
Austria
Rest of Western Europe
Asia Pacific
China
India
Japan
South Korea
Vietnam
Singapore
Australia
Rest of Asia Pacific
Middle East & Africa
Middle East
UAE
Egypt
Saudi Arabia
Qatar
Rest of the Middle East
Africa
Nigeria
South Africa
Rest of Africa
Latin America
Brazil
Argentina
Colombia
Rest of Latin America
Request for Country Level Research Report: Country Level Customization Request
Available Customization
With the given market data, SNS Insider offers customization as per the company’s specific needs. The following customization options are available for the report:
Product Analysis
Criss-Cross segment analysis (e.g. Product X Application)
Product Matrix which gives a detailed comparison of the product portfolio of each company
Geographic Analysis
Additional countries in any of the regions
Company Information
Detailed analysis and profiling of additional market players (Up to five)
The Covered Stent Market size was valued at USD 1.90 billion in 2023 and is expected to reach USD 2.69 billion by 2032 and grow at a CAGR of 3.96% over the forecast period 2024-2032.
The Artificial Tears Market size was USD 3.09 Billion in 2023 and is expected to reach USD 5.94 Billion by 2032 and grow at a CAGR of 7.55% over the forecast period of 2024-2032.
Healthcare Fraud Analytics Market Size was valued at USD 2.36 Billion in 2023 and is expected to reach USD 17.6 Billion by 2032, growing at a CAGR of 25% over the forecast period 2024-2032.
The global antibiotics market size was USD 47.23 Billion in 2023 & is expected to reach USD 65.23 billion by 2032 at a CAGR of 3.70%.
The Brain Computer Interface Market size was valued at USD 2.23 billion in 2023 and is expected to reach USD 8.36 billion by 2032 and grow at a CAGR of 15.81% over the forecast period of 2024-2032.
The Molecular Spectroscopy Market size was valued at USD 7.15 billion in 2023, projected to grow at a 7.15% CAGR, reaching USD 13.28 billion by 2032.
Hi! Click one of our member below to chat on Phone