Get more information on Anesthesia Monitoring Devices Market - Request Sample Report
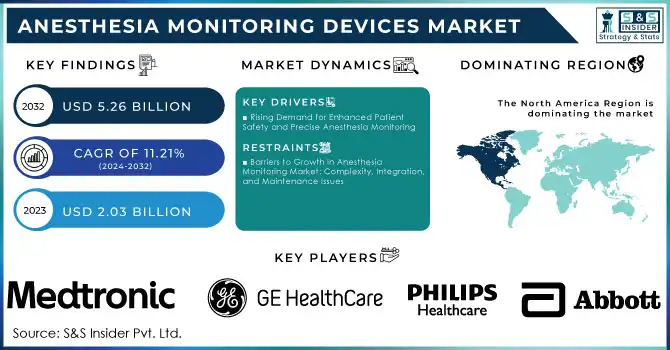
Anesthesia Monitoring Devices Market was valued at USD 2.03 billion in 2023 and is expected to reach USD 5.26 billion by 2032, growing at a CAGR of 11.21% from 2024-2032.
The market for anesthesia monitoring devices is set for considerable expansion as technological advancements improve monitoring functions. Capabilities such as real-time gas analysis, depth-of-anesthesia assessment, and integration with electronic health records allow healthcare professionals to better manage patients throughout intricate procedures. These advancements lessen complications and enhance results, encouraging hospitals and surgical centers to invest further in advanced monitoring technologies. Implementing AI technologies, like Artificial Neural Networks (ANN), which show an accuracy of 83.3%, enhances this trend by increasing monitoring accuracy and ensuring patient safety.
Ambulatory Surgical Centers (ASCs) offer a substantial chance for the expansion of anesthesia monitoring devices. ASCs generate USD 42.2 billion in healthcare savings over one year and USD 4.2 billion in yearly Medicare savings. With the rise of outpatient procedures, these centers require streamlined, effective, and affordable monitoring solutions. Integrated anesthesia workstations that merge anesthesia administration with advanced monitoring capabilities are beautiful because they conserve space and optimize workflows. The move towards outpatient care promotes the use of advanced anesthesia systems, allowing ASCs to deliver safe, high-quality care while effectively managing costs.
The adoption of AI in healthcare is minimizing the time doctors allocate to administrative responsibilities and enhancing efficiency. Before adopting AI, physicians dedicated 50% of their time to direct patient care. After implementation, this increased to 67%, resulting in a 20% decrease in time allocated to administrative tasks. This transition enhances operational efficiency and enables healthcare providers to concentrate more on patient care. As AI advances, anesthesia monitoring equipment will gain from improved predictive analytics, increasing both safety and quality of care.
DRIVERS
Rising Demand for Enhanced Patient Safety and Precise Anesthesia Monitoring
To meet the increasing need for patient safety in anesthesia monitoring, ongoing advancements in technologies like AI and improved sensors are crucial. Emphasizing personalized medicine can assist in customizing monitoring to meet specific patient requirements, enhancing accuracy. The incorporation of machine learning is essential for early complication prediction, enabling proactive treatment. Moreover, more stringent safety regulations and governmental backing for advanced monitoring systems are encouraging healthcare providers to invest in these technologies to guarantee the best results and minimize complications during procedures.
RESTRAINTS
Barriers to Growth in Anesthesia Monitoring Market: Complexity, Integration, and Maintenance Issues
The market for anesthesia monitoring devices encounters various limitations that could affect its expansion. The significant upfront expenses for advanced monitoring systems may restrict their use, especially in smaller healthcare institutions. The intricacy of these devices necessitates skilled workers, and a lack of qualified technicians can pose a challenge. Moreover, regulatory hurdles, such as rigorous approval procedures, may postpone the launch of groundbreaking solutions. Challenges with integrating into current infrastructure, along with the continuous maintenance and support of these systems, can additionally limit market growth, particularly in resource-limited environments.
BY END USE
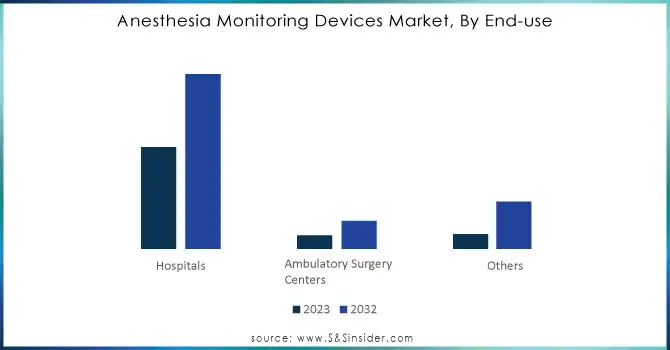
In 2023, hospitals led the market for anesthesia monitoring devices, representing approximately 74% of the revenue, owing to their high patient volumes and the demand for sophisticated, ongoing monitoring during intricate surgical procedures. These organizations usually possess enhanced access to resources, allowing them to purchase advanced monitoring tools. Conversely, Ambulatory Surgery Centers (ASCs) are projected to experience the highest CAGR of 14.31% from 2024 to 2032, fueled by the rising need for minimally invasive procedures and affordable healthcare options. The increase in outpatient surgeries and reduced recovery periods in ASCs drive this swift expansion, as these facilities emphasize enhancing efficiency and patient results. As per STTS, the shift towards outpatient care is anticipated to enhance the use of anesthesia monitoring devices in ASCs.
Need any customization research on Anesthesia Monitoring Devices Market - Enquiry Now
BY PRODUCT
TIn 2023, advanced anesthesia monitors led the anesthesia monitoring devices market, accounting for roughly 51% of the revenue, owing to their capability of delivering detailed, real-time data to enhance patient safety and clinical results. These monitors provide enhanced functionalities such as multi-parameter monitoring, tracking the depth of anesthesia, and wireless connectivity, rendering them crucial for intricate surgeries. The segment is projected to experience the highest CAGR of 12.23% from 2024 to 2032, fueled by the rising need for more accurate and dependable monitoring in hospitals and outpatient facilities. As per STTS, the increase in patient safety worries and the heightened inclination towards minimally invasive techniques will boost the implementation of sophisticated anesthesia monitoring systems.
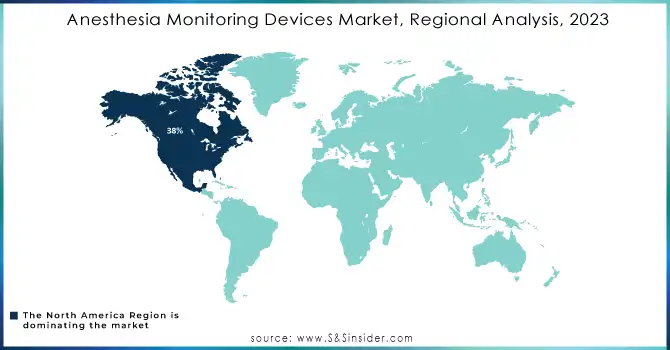
In 2023, North America led the anesthesia monitoring devices market, contributing to approximately 38% of the revenue, mainly because of its advanced healthcare infrastructure, significant healthcare expenditures, and a strong commitment to patient safety. This area hosts top manufacturers and early users of cutting-edge medical technologies, establishing it as an essential market for anesthesia monitoring devices. Moreover, many surgeries that necessitate advanced anesthesia monitoring and the presence of established healthcare systems enhance its prevalence.
North America is anticipated to experience the highest CAGR of 12.00% from 2024 to 2032, fueled by the rising demand for minimally invasive procedures and improved monitoring technologies. The area's older demographic, increasing incidence of chronic illnesses, and technological progress are anticipated to drive market expansion. As per STTS, the move towards outpatient treatments and the constant advancement of next-gen monitoring systems will further enhance the acceptance of anesthesia monitoring devices in North America.
LATEST NEWS -
Medtronic's BIS™ Advance monitor obtained FDA 510(k) clearance in 2024, providing a revamped interface for improved brain monitoring. This tool assists anesthesia practitioners in tailoring dosages, enhancing patient results by tracking the depth of anesthesia with greater precision.
In 2024, GE HealthCare and Hartford HealthCare extended their seven-year collaboration to enhance patient care in Connecticut. The partnership aims to improve patient monitoring, anesthesia, and maternal-infant care using advanced AI-driven medical devices.
KEY PLAYERS
Medtronic (Datex-Ohmeda S/5 Anesthesia Monitor, Capnograph Monitoring System)
GE Healthcare (Carestation 650 Anesthesia System, Datex-Ohmeda S/5 Anesthesia Monitor)
Philips Healthcare (IntelliVue MX40, IntelliVue MP5 Anesthesia Monitor)
Drägerwerk AG & Co. KGaA (Fabius Anesthesia Workstation, Perseus A500 Anesthesia Machine)
Masimo Corporation (Rad-97 Pulse CO-Oximeter, Root Patient Monitoring Platform)
Mindray Medical International Limited (A3 Anesthesia Monitor, BeneView T8 Patient Monitor)
Nihon Kohden Corporation (NM-8000 Anesthesia Monitor, Life Scope G5 Patient Monitor)
Schiller AG (Custo Screen 100, Custo Monitor 5000)
Becton, Dickinson and Company (BD) (BD Nexiva™, BD Alaris™ System)
Fukuda Denshi Co. Ltd. (FCP-8100 Anesthesia Monitor, DS-5200 Patient Monitor)
Infinium Medical, Inc. (Anesthesia Monitor 5000, Infinium Patient Monitor)
Koninklijke Philips N.V. (IntelliVue MP5, IntelliVue MX40)
Siemens Healthineers (SC7000 Anesthesia Monitor, SOMATOM Definition Edge CT Scanner)
Abbott Laboratories (Alinity m System, POCcelerate Anesthesia Monitor)
Honeywell International (Honeywell Sensing and Internet of Things Solutions, Series 3000 Anesthesia Monitor)
Mindray (BeneView T8, A3 Anesthesia Monitor)
Fujifilm Holdings Corporation (Fujifilm SonoSite Edge II, SonoSite M-Turbo Ultrasound)
Beijing Xiyuan Technology (XH-8020 Anesthesia Monitor, XH-8030 Patient Monitor)
Cairnry Corporation (AeroSync Monitoring System, AEROSIM Anesthesia Simulator)
Biolight (BL-8200 Anesthesia Monitor, BL-3200 Multi-Parameter Patient Monitor)
Toshiba Medical Systems (Aquilion One, Anesthesia Workstation MA-3000)
Carestream Health (Carestream OnSight 3D Extremity System, Carestream PACS for Anesthesia)
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | USD 2.03 Billion |
| Market Size by 2032 | USD 5.26 Billion |
| CAGR | CAGR of 11.21% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Product (Basic Anesthesia Monitor, Integrated Anesthesia Workstation, Advanced Anesthesia Monitor) • By End-use (Hospitals, Ambulatory Surgery Centers, Others) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | Medtronic, GE Healthcare, Philips Healthcare, Drägerwerk AG & Co. KGaA, Masimo Corporation, Mindray Medical International Limited, Nihon Kohden Corporation, Schiller AG, Becton, Dickinson and Company (BD), Fukuda Denshi Co. Ltd., Infinium Medical, Inc., Koninklijke Philips N.V., Siemens Healthineers, Abbott Laboratories, Honeywell International, Mindray, Fujifilm Holdings Corporation, Beijing Xiyuan Technology, Cairnry Corporation, Biolight, Toshiba Medical Systems, Carestream Health. |
| Key Drivers | • Rising Demand for Enhanced Patient Safety and Precise Anesthesia Monitoring |
| RESTRAINTS | • Barriers to Growth in Anesthesia Monitoring Market: Complexity, Integration, and Maintenance Issues |
Anesthesia Monitoring Devices Market was valued at USD 2.03 billion in 2023 and is expected to reach USD 5.26 billion by 2032, growing at a CAGR of 11.21% from 2024-2032.
Rising demand for enhanced patient safety, precise monitoring, and advancements in AI and sensor technologies are key market drivers.
Hospitals led the market, accounting for approximately 74% of the revenue share.
Advanced anesthesia monitors dominated the market with around 51% of the revenue, due to their comprehensive, real-time monitoring capabilities.
North America is expected to grow at a CAGR of 12.00% from 2024 to 2032.
Table of Content
1. Introduction
1.1 Market Definition
1.2 Scope (Inclusion and Exclusions)
1.3 Research Assumptions
2. Executive Summary
2.1 Market Overview
2.2 Regional Synopsis
2.3 Competitive Summary
3. Research Methodology
3.1 Top-Down Approach
3.2 Bottom-up Approach
3.3. Data Validation
3.4 Primary Interviews
4. Market Dynamics Impact Analysis
4.1 Market Driving Factors Analysis
4.1.2 Drivers
4.1.2 Restraints
4.1.3 Opportunities
4.1.4 Challenges
4.2 PESTLE Analysis
4.3 Porter’s Five Forces Model
5. Statistical Insights and Trends Reporting
5.1 Incidence and Prevalence (2023)
5.2 Prescription Trends, (2023), by Region
5.3 Device Volume, by Region (2020-2032)
5.4 Healthcare Spending, by region, (Government, Commercial, Private, Out-of-Pocket), 2023
6. Competitive Landscape
6.1 List of Major Companies, By Region
6.2 Market Share Analysis, By Region
6.3 Product Benchmarking
6.3.1 Product specifications and features
6.3.2 Pricing
6.4 Strategic Initiatives
6.4.1 Marketing and promotional activities
6.4.2 Distribution and supply chain strategies
6.4.3 Expansion plans and new product launches
6.4.4 Strategic partnerships and collaborations
6.5 Technological Advancements
6.6 Market Positioning and Branding
7. Anesthesia Monitoring Devices Market Segmentation, by Product
7.1 Chapter Overview
7.2 Basic Anesthesia Monitor
7.2.1 Basic Anesthesia Monitor Market Trends Analysis (2020-2032)
7.2.2 Basic Anesthesia Monitor Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3 Integrated Anesthesia Workstation
7.3.1 Integrated Anesthesia Workstation Market Trends Analysis (2020-2032)
7.3.2 Integrated Anesthesia Workstation Market Size Estimates and Forecasts to 2032 (USD Billion)
7.4 Advanced Anesthesia Monitor
7.4.1 Advanced Anesthesia Monitor Market Trends Analysis (2020-2032)
7.4.2 Advanced Anesthesia Monitor Market Size Estimates and Forecasts to 2032 (USD Billion)
8. Anesthesia Monitoring Devices Market Segmentation, by End Use
8.1 Chapter Overview
8.2 Hospitals
8.2.1 Hospitals Market Trends Analysis (2020-2032)
8.2.2 Hospitals Market Size Estimates and Forecasts to 2032 (USD Billion)
8.3 Ambulatory Surgical Centers
8.3.1 Ambulatory Surgical Centers Market Trends Analysis (2020-2032)
8.3.2 Ambulatory Surgical Centers Market Size Estimates and Forecasts to 2032 (USD Billion)
8.4 Others
8.4.1 Others Market Trends Analysis (2020-2032)
8.4.2 Others Market Size Estimates and Forecasts to 2032 (USD Billion)
9. Regional Analysis
9.1 Chapter Overview
9.2 North America
9.2.1 Trends Analysis
9.2.2 North America Anesthesia Monitoring Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.2.3 North America Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.2.4 North America Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.2.5 USA
9.2.5.1 USA Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.2.5.2 USA Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.2.6 Canada
9.2.6.1 Canada Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.2.6.2 Canada Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.2.7 Mexico
9.2.7.1 Mexico Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.2.7.2 Mexico Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.3 Europe
9.3.1 Eastern Europe
9.3.1.1 Trends Analysis
9.3.1.2 Eastern Europe Anesthesia Monitoring Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.3.1.3 Eastern Europe Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.3.1.4 Eastern Europe Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.3.1.5 Poland
9.3.1.5.1 Poland Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.3.1.5.2 Poland Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.3.1.6 Romania
9.3.1.6.1 Romania Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.3.1.6.2 Romania Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.3.1.7 Hungary
9.3.1.7.1 Hungary Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.3.1.7.2 Hungary Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.3.1.8 Turkey
9.3.1.8.1 Turkey Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.3.1.8.2 Turkey Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.3.1.9 Rest of Eastern Europe
9.3.1.9.1 Rest of Eastern Europe Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.3.1.9.2 Rest of Eastern Europe Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.3.2 Western Europe
9.3.2.1 Trends Analysis
9.3.2.2 Western Europe Anesthesia Monitoring Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.3.2.3 Western Europe Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.3.2.4 Western Europe Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.3.2.5 Germany
9.3.2.5.1 Germany Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.3.2.5.2 Germany Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.3.2.6 France
9.3.2.6.1 France Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.3.2.6.2 France Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.3.2.7 UK
9.3.2.7.1 UK Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.3.2.7.2 UK Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.3.2.8 Italy
9.3.2.8.1 Italy Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.3.2.8.2 Italy Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.3.2.9 Spain
9.3.2.9.1 Spain Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.3.2.9.2 Spain Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.3.2.10 Netherlands
9.3.2.10.1 Netherlands Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.3.2.10.2 Netherlands Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.3.2.11 Switzerland
9.3.2.11.1 Switzerland Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.3.2.11.2 Switzerland Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.3.2.12 Austria
9.3.2.12.1 Austria Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.3.2.12.2 Austria Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.3.2.13 Rest of Western Europe
9.3.2.13.1 Rest of Western Europe Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.3.2.13.2 Rest of Western Europe Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.4 Asia Pacific
9.4.1 Trends Analysis
9.4.2 Asia Pacific Anesthesia Monitoring Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.4.3 Asia Pacific Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.4.4 Asia Pacific Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.4.5 China
9.4.5.1 China Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.4.5.2 China Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.4.6 India
9.4.5.1 India Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.4.5.2 India Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.4.5 Japan
9.4.5.1 Japan Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.4.5.2 Japan Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.4.6 South Korea
9.4.6.1 South Korea Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.4.6.2 South Korea Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.4.7 Vietnam
9.4.7.1 Vietnam Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.2.7.2 Vietnam Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.4.8 Singapore
9.4.8.1 Singapore Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.4.8.2 Singapore Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.4.9 Australia
9.4.9.1 Australia Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.4.9.2 Australia Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.4.10 Rest of Asia Pacific
9.4.10.1 Rest of Asia Pacific Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.4.10.2 Rest of Asia Pacific Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.5 Middle East and Africa
9.5.1 Middle East
9.5.1.1 Trends Analysis
9.5.1.2 Middle East Anesthesia Monitoring Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.5.1.3 Middle East Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.5.1.4 Middle East Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.5.1.5 UAE
9.5.1.5.1 UAE Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.5.1.5.2 UAE Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.5.1.6 Egypt
9.5.1.6.1 Egypt Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.5.1.6.2 Egypt Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.5.1.7 Saudi Arabia
9.5.1.7.1 Saudi Arabia Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.5.1.7.2 Saudi Arabia Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.5.1.8 Qatar
9.5.1.8.1 Qatar Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.5.1.8.2 Qatar Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.5.1.9 Rest of Middle East
9.5.1.9.1 Rest of Middle East Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.5.1.9.2 Rest of Middle East Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.5.2 Africa
9.5.2.1 Trends Analysis
9.5.2.2 Africa Anesthesia Monitoring Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.5.2.3 Africa Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.5.2.4 Africa Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.5.2.5 South Africa
9.5.2.5.1 South Africa Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.5.2.5.2 South Africa Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.5.2.6 Nigeria
9.5.2.6.1 Nigeria Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.5.2.6.2 Nigeria Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.5.2.7 Rest of Africa
9.5.2.7.1 Rest of Africa Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.5.2.7.2 Rest of Africa Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.6 Latin America
9.6.1 Trends Analysis
9.6.2 Latin America Anesthesia Monitoring Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
9.6.3 Latin America Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.6.4 Latin America Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.6.5 Brazil
9.6.5.1 Brazil Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.6.5.2 Brazil Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.6.6 Argentina
9.6.6.1 Argentina Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.6.6.2 Argentina Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.6.7 Colombia
9.6.7.1 Colombia Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.6.7.2 Colombia Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
9.6.8 Rest of Latin America
9.6.8.1 Rest of Latin America Anesthesia Monitoring Devices Market Estimates and Forecasts, by Product (2020-2032) (USD Billion)
9.6.8.2 Rest of Latin America Anesthesia Monitoring Devices Market Estimates and Forecasts, by End Use (2020-2032) (USD Billion)
10. Company Profiles
10.1 Medtronic
10.1.1 Company Overview
10.1.2 Financial
10.1.3 Products/ Services Offered
110.1.4 SWOT Analysis
10.2 GE Healthcare
10.2.1 Company Overview
10.2.2 Financial
10.2.3 Products/ Services Offered
10.2.4 SWOT Analysis
10.3 Philips Healthcare
10.3.1 Company Overview
10.3.2 Financial
10.3.3 Products/ Services Offered
10.3.4 SWOT Analysis
10.4 Drägerwerk AG & Co. KGaA
10.4.1 Company Overview
10.4.2 Financial
10.4.3 Products/ Services Offered
10.4.4 SWOT Analysis
10.5 Masimo Corporation
10.5.1 Company Overview
10.5.2 Financial
10.5.3 Products/ Services Offered
10.5.4 SWOT Analysis
10.6 Mindray Medical International Limited
10.6.1 Company Overview
10.6.2 Financial
10.6.3 Products/ Services Offered
10.6.4 SWOT Analysis
10.7 Nihon Kohden Corporation
10.7.1 Company Overview
10.7.2 Financial
10.7.3 Products/ Services Offered
10.7.4 SWOT Analysis
10.8 Schiller AG
10.8.1 Company Overview
10.8.2 Financial
10.8.3 Products/ Services Offered
10.8.4 SWOT Analysis
10.9 Becton, Dickinson and Company (BD)
10.9.1 Company Overview
10.9.2 Financial
10.9.3 Products/ Services Offered
10.9.4 SWOT Analysis
10.10 Infinium Medical, Inc
10.9.1 Company Overview
10.9.2 Financial
10.9.3 Products/ Services Offered
10.9.4 SWOT Analysis
11. Use Cases and Best Practices
12. Conclusion
An accurate research report requires proper strategizing as well as implementation. There are multiple factors involved in the completion of good and accurate research report and selecting the best methodology to compete the research is the toughest part. Since the research reports we provide play a crucial role in any company’s decision-making process, therefore we at SNS Insider always believe that we should choose the best method which gives us results closer to reality. This allows us to reach at a stage wherein we can provide our clients best and accurate investment to output ratio.
Each report that we prepare takes a timeframe of 350-400 business hours for production. Starting from the selection of titles through a couple of in-depth brain storming session to the final QC process before uploading our titles on our website we dedicate around 350 working hours. The titles are selected based on their current market cap and the foreseen CAGR and growth.
The 5 steps process:
Step 1: Secondary Research:
Secondary Research or Desk Research is as the name suggests is a research process wherein, we collect data through the readily available information. In this process we use various paid and unpaid databases which our team has access to and gather data through the same. This includes examining of listed companies’ annual reports, Journals, SEC filling etc. Apart from this our team has access to various associations across the globe across different industries. Lastly, we have exchange relationships with various university as well as individual libraries.

Step 2: Primary Research
When we talk about primary research, it is a type of study in which the researchers collect relevant data samples directly, rather than relying on previously collected data. This type of research is focused on gaining content specific facts that can be sued to solve specific problems. Since the collected data is fresh and first hand therefore it makes the study more accurate and genuine.
We at SNS Insider have divided Primary Research into 2 parts.
Part 1 wherein we interview the KOLs of major players as well as the upcoming ones across various geographic regions. This allows us to have their view over the market scenario and acts as an important tool to come closer to the accurate market numbers. As many as 45 paid and unpaid primary interviews are taken from both the demand and supply side of the industry to make sure we land at an accurate judgement and analysis of the market.
This step involves the triangulation of data wherein our team analyses the interview transcripts, online survey responses and observation of on filed participants. The below mentioned chart should give a better understanding of the part 1 of the primary interview.

Part 2: In this part of primary research the data collected via secondary research and the part 1 of the primary research is validated with the interviews from individual consultants and subject matter experts.
Consultants are those set of people who have at least 12 years of experience and expertise within the industry whereas Subject Matter Experts are those with at least 15 years of experience behind their back within the same space. The data with the help of two main processes i.e., FGDs (Focused Group Discussions) and IDs (Individual Discussions). This gives us a 3rd party nonbiased primary view of the market scenario making it a more dependable one while collation of the data pointers.
Step 3: Data Bank Validation
Once all the information is collected via primary and secondary sources, we run that information for data validation. At our intelligence centre our research heads track a lot of information related to the market which includes the quarterly reports, the daily stock prices, and other relevant information. Our data bank server gets updated every fortnight and that is how the information which we collected using our primary and secondary information is revalidated in real time.

Step 4: QA/QC Process
After all the data collection and validation our team does a final level of quality check and quality assurance to get rid of any unwanted or undesired mistakes. This might include but not limited to getting rid of the any typos, duplication of numbers or missing of any important information. The people involved in this process include technical content writers, research heads and graphics people. Once this process is completed the title gets uploader on our platform for our clients to read it.
Step 5: Final QC/QA Process:
This is the last process and comes when the client has ordered the study. In this process a final QA/QC is done before the study is emailed to the client. Since we believe in giving our clients a good experience of our research studies, therefore, to make sure that we do not lack at our end in any way humanly possible we do a final round of quality check and then dispatch the study to the client.
Key Segments:
By Product
Basic Anesthesia Monitor
Integrated Anesthesia Workstation
Advanced Anesthesia Monitor
By End-use
Hospitals
Ambulatory Surgery Centers
Others
Request for Segment Customization as per your Business Requirement: Segment Customization Request
Regional Coverage:
North America
US
Canada
Mexico
Europe
Eastern Europe
Poland
Romania
Hungary
Turkey
Rest of Eastern Europe
Western Europe
Germany
France
UK
Italy
Spain
Netherlands
Switzerland
Austria
Rest of Western Europe
Asia Pacific
China
India
Japan
South Korea
Vietnam
Singapore
Australia
Rest of Asia Pacific
Middle East & Africa
Middle East
UAE
Egypt
Saudi Arabia
Qatar
Rest of the Middle East
Africa
Nigeria
South Africa
Rest of Africa
Latin America
Brazil
Argentina
Colombia
Rest of Latin America
Request for Country Level Research Report: Country Level Customization Request
Available Customization
With the given market data, SNS Insider offers customization as per the company’s specific needs. The following customization options are available for the report:
Product Analysis
Criss-Cross segment analysis (e.g. Product X Application)
Product Matrix which gives a detailed comparison of the product portfolio of each company
Geographic Analysis
Additional countries in any of the regions
Company Information
Detailed analysis and profiling of additional market players (Up to five)
The Single-use Bioprocessing Market size was USD 26.8 billion in 2023 and is expected to reach USD 100.9 billion by 2032 at a CAGR of 15.9%.
The Pediatric Cancer Biomarker Market was valued at USD 830.41 Million in 2023 and is projected to reach USD 1635.68 Million by 2032 with a growing CAGR of 7.84%.
The Molecular Diagnostics Market Size was USD 15.35 Billion in 2023, and expected to reach USD 32.37 Billion by 2032, and grow at a CAGR of 9.07%.
The carrier screening market valued at USD 2.26 Billion in 2023, projected to reach USD 11.44 Billion by 2032, growing at a CAGR of 19.76% from 2024–2032.
The Non-Invasive Prenatal Testing (NIPT) Market is expected to reach USD 17.75 Bn by 2031 and was valued at USD 6.4 Bn in 2023, and grow at a CAGR of 13.6% over the forecast period of 2024-2031.
The Diabetic Nephropathy Market size was valued at USD 2.33 Billion in 2023 and is expected to reach USD 3.72 Billion By 2031 and grow at a CAGR of 6.04% over the forecast period of 2024-2031.
Hi! Click one of our member below to chat on Phone