Get more information on 3D Printing Medical Devices Market - Request Sample Report
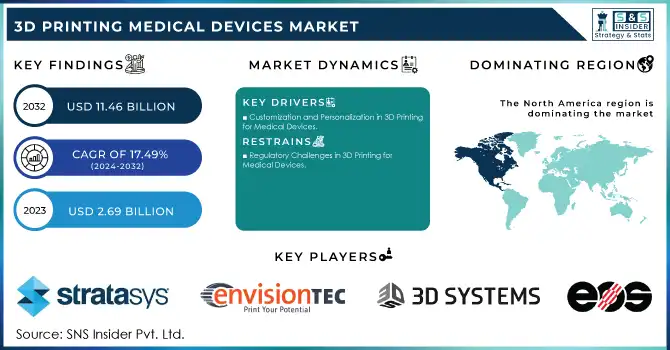
The 3D Printing Medical Devices Market Size was valued at USD 2.69 billion in 2023 and is expected to reach USD 11.46 billion by 2032 and grow at a CAGR of 17.49% over the forecast period 2024-2032.
The 3D printing technology in medical devices is rapidly advancing, driven by its ability to provide customized and highly efficient healthcare solutions. In 2023, over 100,000 3D-printed medical devices were approved by the U.S. Food and Drug Administration (FDA), showcasing the technology's growing acceptance and integration into healthcare systems. The FDA’s recent clearance of 3D-printed devices, including the V-PRO maX 2 Low-Temperature Sterilization System, designed specifically for 3D-printed surgical tools, highlights the expanding role of 3D printing in surgical procedures. This system is a notable step forward in ensuring the safety and usability of 3D-printed medical instruments.
One of the most significant areas of impact is in personalized medicine, particularly in orthopedics and dentistry. Companies like Materialise and Stratasys are producing customized joint replacements and prosthetics that offer better anatomical fits for patients, improving post-surgery recovery. In dental care, Formlabs has made strides in providing 3D-printed crowns, bridges, and dentures, enabling faster and more precise treatments compared to traditional methods. Additionally, the development of 3D-printed titanium spinal interbody fusion devices and nerve repair implants by companies such as NanoHive Medical demonstrates the far-reaching applications of 3D printing in addressing complex medical needs.
The growth of bioprinting is another transformative development. For example, Organovo has been at the forefront of 3D bioprinting, producing liver tissue for drug testing, with ongoing efforts to create functional human tissues for therapeutic purposes. Regulatory advancements also support this growth, with the FDA issuing guidance for 3D-printed devices and ensuring they meet the necessary safety standards. Moreover, healthcare institutions are increasingly utilizing 3D-printed anatomical models for pre-surgical planning. For instance, the University of Michigan uses 3D-printed models to assist surgeons in visualizing and planning complex surgeries, significantly reducing surgery times.
While challenges such as regulatory hurdles, material compatibility, and quality control remain, ongoing technological and regulatory advancements continue to shape a future where 3D printing plays an integral role in improving patient outcomes and making healthcare more personalized and efficient.
Drivers
Customization and Personalization in 3D Printing for Medical Devices
One of the key advantages of 3D printing in the medical field is its ability to offer customization and personalization for medical devices, prosthetics, and implants. Traditional manufacturing methods often require standard sizes and shapes, limiting the ability to fully address individual patient needs. In contrast, 3D printing enables the creation of devices that are specifically tailored to a patient’s unique anatomy, resulting in better fitting, more comfortable, and highly functional medical solutions. In orthopedics, for example, 3D printing allows for the production of customized joint replacements and implants that match the patient's bone structure more precisely, improving surgical outcomes and reducing recovery time. Similarly, in dental care, 3D printing is used to create personalized crowns, bridges, and dentures that offer a more accurate fit compared to conventional methods, leading to improved patient satisfaction. This level of customization not only enhances patient comfort but also minimizes the risk of complications, as the devices are designed to suit individual medical needs. Additionally, personalized medical devices enable healthcare providers to offer more effective treatment plans, improving the overall quality of care. As 3D printing technology advances, the ability to customize medical devices will continue to transform healthcare practices.
Advancements in Bioprinting Fueling the Future of Medical Innovation
Bioprinting has emerged as one of the most exciting advancements in 3D printing technology, significantly transforming the healthcare landscape. This innovative process involves using 3D printers to create complex biological tissues and structures using living cells as "inks." With ongoing improvements in bioprinting techniques, researchers have made substantial progress in developing functional human tissues, which can be used for drug testing, disease modeling, and regenerative medicine.
Bioprinted tissues offer a promising alternative to animal testing, providing more accurate and ethical models for drug development. By printing human-like tissues, pharmaceutical companies can better predict how drugs will interact with human cells, leading to faster and more effective treatments. Additionally, bioprinting holds immense potential for regenerative medicine. Scientists are working towards printing organs such as kidneys, livers, and hearts, to address the global organ transplant shortage.
Cost-Effective Manufacturing in 3D Printing for Medical Devices
One of the key advantages driving the adoption of 3D printing in the medical device industry is its cost-effectiveness. Traditional manufacturing methods for medical devices often involve expensive molds, tooling, and large-scale production processes. These costs can be prohibitive, particularly when producing low volumes or customized devices. In contrast, 3D printing eliminates the need for complex molds and tooling, making it an ideal solution for producing intricate, customized medical devices at a lower cost.
The ability to print medical devices directly from digital files allows manufacturers to create prototypes and final products without the overhead associated with traditional manufacturing methods. This process is especially beneficial for producing one-off devices or small batches of custom implants, prosthetics, or surgical tools, where traditional methods would be too costly. For example, 3D printing enables orthopedic surgeons to create custom-fit implants and prosthetics for individual patients, minimizing the need for mass production runs.
Restraints
Regulatory Challenges in 3D Printing for Medical Devices
The regulatory landscape for 3D-printed medical devices remains fragmented, with different regions and countries having their specific approval processes and standards. This lack of harmonization can lead to delays in bringing new products to market and creates challenges for manufacturers navigating varying compliance requirements globally.
Challenges in Material Selection for 3D-printed Medical Devices
The ongoing development of suitable biocompatible, durable, and long-lasting materials for medical devices remains a key challenge in the 3D printing industry, limiting the scope and effectiveness of these devices in healthcare applications.
By Component
In 2023, the 3D Printers segment dominated the market, holding a substantial share due to the growing demand for custom medical devices, implants, and surgical tools. The adoption of 3D printers in healthcare has been driven by the need for rapid prototyping, personalization of medical devices, and the increasing trend towards minimally invasive surgeries. 3D printers enable the production of patient-specific solutions, making them ideal for creating customized prosthetics, implants, and surgical instruments. The segment held approximately 40.0% of the market share in 2023.
The 3D Bioprinters segment is the fastest-growing within the equipment category, gaining significant traction due to advancements in bioprinting technology. These printers are crucial for creating tissue models, and organ prototypes, and supporting regenerative medicine and drug testing. The growing applications of bioprinting in research and the development of patient-specific biological solutions are key drivers behind the growth of this segment.
By Type
The Surgical Guides segment emerged as the leading category in 2023, accounting for a major share of the market. Surgical guides are extensively used in orthopedics, dental procedures, and craniomaxillofacial surgeries to enhance precision and reduce human error during operations. These guides help plan and execute surgeries more effectively, contributing to faster recovery times for patients. The Surgical Guides segment dominated the market with a share of 35.0% in 2023.
Among surgical guides, Orthopedic Guides experienced the fastest growth, driven by the increasing number of orthopedic surgeries and the demand for customized solutions. These guides help surgeons perform precise procedures, such as joint replacements, using personalized models based on patient anatomy, improving surgical outcomes.
In 2023, North America dominated the 3D printing medical devices market, accounting for the largest share. The region's leadership can be attributed to the strong presence of key players, well-established healthcare infrastructure, and significant investments in advanced technologies. The United States, in particular, is a major contributor, with high adoption rates of 3D printing for personalized medicine, prosthetics, implants, and surgical planning. The regulatory environment, including support from the U.S. FDA for 3D-printed medical devices, has further fueled market growth.
Europe also held a significant share of the market, driven by countries like Germany, the UK, and France. These nations have advanced healthcare systems and robust research capabilities, particularly in 3D bioprinting, surgical guides, and customized implants. The region benefits from collaborative efforts between research institutions, healthcare providers, and industry leaders, driving the development and commercialization of 3D printing technologies for medical use.
Asia-Pacific is the fastest-growing region, with countries like China, Japan, and India showing increased adoption of 3D printing in healthcare. The rapid advancements in healthcare infrastructure, coupled with a growing focus on medical device manufacturing, are key drivers in this region. China, in particular, is investing heavily in 3D printing technologies for medical applications, positioning itself as a leader in bioprinting and custom medical solutions.
Get Customized Report as per Your Business Requirement - Enquiry Now
J750 Digital Anatomy Printer
F370 CR
2. EnvisionTEC
Perfactory P4K
3D-Bioplotter
3. 3D Systems, Inc.
ProX DMP 320
Figure 4
4. EOS GmbH
EOSINT M 280
EOS P 396
5. Renishaw plc
RenAM 500Q
Additive Manufacturing System
6. GE Additive
Arcam EBM
Concept Laser M2 Cusing
7. Desktop Metal, Inc.
Studio System
8. CELLINK
BIO X
INKREDIBLE
9. Materialise
Materialise Magics
Materialise Mimics
10. 3T Additive Manufacturing Ltd.
3T Metal 3D Printing
11. General Electric Company
Arcam Q10
12. Carbon, Inc.
Carbon M1
13. Prodways Group
ProMaker L6000
14. SLM Solutions
SLM 280
15. Organovo Holdings Inc.
NovoGen MMX Bioprinter
16. FIT AG
FIT Additive Manufacturing
17. Wacker Chemie AG
Silicone-based 3D printing materials
18. Dentsply Sirona
SIRONA CEREC
19. DWS Systems SRL
XFAB 2000
20. Roland DG
DWX-52DC
21. HP, Inc.
HP Jet Fusion 3D
22. regenHU
3D-Bioprinting Platform
23. Fluicell
Bioprinting System
24. Proto Labs
Protolabs 3D Printing
25. GESIM
Bio 3D Printer
26. Triastek
3D Printed Drug Delivery Systems
27. Inventia
Rastrum
28. FabRx
FDM Printers
29. Apprecia Pharmaceuticals
3D-Printed Tablets
In Oct 2024, Biological Lattice Industries (BLI), a U.S.-based biofabrication company, raised USD 1.8 million in pre-seed funding to advance its AI-powered software that streamlines the biofabrication process. The investment will support the company’s efforts to simplify biofabrication for the global research and development market.
In Oct 2024, LuxCreo entered the USD 500 billion medical device market, introducing same-day and scalable manufacturing for its EMA 3D sleep apnea device. This move is set to revolutionize production speed and scalability within the industry.
In Aug 2024, NanoHive Medical secured USD 7 million in Series C funding to expand its US commercial team, enhance its Hive portfolio of Soft Titanium spinal devices, and enter international markets. The investment will also support the development of new products and smart sensor implant technologies.
| Report Attributes | Details |
|---|---|
| Market Size in 2023 | USD 2.69 billion |
| Market Size by 2032 | USD 11.46 Billion |
| CAGR | CAGR of 17.49% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Report Scope & Coverage | Market Size, Segments Analysis, Competitive Landscape, Regional Analysis, DROC & SWOT Analysis, Forecast Outlook |
| Key Segments | • By Component (Software and Services, Equipment, 3D Printers, 3D Bioprinters, Biomaterials) • By Type (Surgical Guides, Dental Guides, Craniomaxillofacial Guides, Orthopedic Guides, Surgical Instruments, Retractors) • By Technology (Electron Beam Melting (EBM) Technology, Laser Beam Melting (LBM) Technology, Direct Metal Laser Sintering (DMLS), Selective Laser Melting (SLM), Selective Laser Sintering (SLS), Photopolymerization) |
| Regional Analysis/Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East], Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia, Rest of Latin America) |
| Company Profiles | Stratasys Ltd., EnvisionTEC, 3D Systems, Inc., EOS GmbH, Renishaw plc, GE Additive, Desktop Metal, Inc., CELLINK, Materialise, 3T Additive Manufacturing Ltd., General Electric Company, Carbon, Inc., Prodways Group, SLM Solutions, Organovo Holdings Inc., FIT AG, Wacker Chemie AG, Dentsply Sirona, DWS Systems SRL, Roland DG, HP, Inc., regenHU, Fluicell, Proto Labs, GESIM, Triastek, Inventia, FabRx, Apprecia Pharmaceuticals |
| Key Drivers | • Customization and Personalization in 3D Printing for Medical Devices • Advancements in Bioprinting Fueling the Future of Medical Innovation • Cost-Effective Manufacturing in 3D Printing for Medical Devices |
| Restraints | • Regulatory Challenges in 3D Printing for Medical Devices • Challenges in Material Selection for 3D-printed Medical Devices |
Ans:- The 3D Printing Medical Devices Market Size was valued at USD 2.69 billion in 2023.
Stratasys Ltd., EnvisionTEC, 3D Systems, Inc., EOS GmbH, Renishaw plc, GE Additive, Desktop Metal, Inc., CELLINK, Materialise, 3T Additive Manufacturing Ltd., General Electric Company, Carbon, Inc., Prodways Group, SLM Solutions, Organovo Holdings Inc., FIT AG, Wacker Chemie AG, Dentsply Sirona, DWS Systems SRL, Roland DG, HP, Inc., regenHU, Fluicell, Proto Labs, GESIM, Triastek, Inventia, FabRx, Apprecia Pharmaceuticals are the key players of the 3D Printing Medical Devices Market.
Ans:- The 3D Printing Medical Devices Market is growing at a CAGR of 17.49% over the forecast period 2024-2032.
Customization and personalization in 3d printing for medical devices propelling the market growth.
Regulatory Challenges in 3D Printing for Medical Devices
Challenges in Material Selection for 3D-printed Medical Devices
Table of Contents
1. Introduction
1.1 Market Definition
1.2 Scope (Inclusion and Exclusions)
1.3 Research Assumptions
2. Executive Summary
2.1 Market Overview
2.2 Regional Synopsis
2.3 Competitive Summary
3. Research Methodology
3.1 Top-Down Approach
3.2 Bottom-up Approach
3.3. Data Validation
3.4 Primary Interviews
4. Market Dynamics Impact Analysis
4.1 Market Driving Factors Analysis
4.1.1 Drivers
4.1.2 Restraints
4.1.3 Opportunities
4.1.4 Challenges
4.2 PESTLE Analysis
4.3 Porter’s Five Forces Model
5. Statistical Insights and Trends Reporting
5.1 Incidence and Prevalence (2023)
5.2 Prescription Trends (2023), by Region
5.3 Device Volume, by Region (2020-2032)
5.4 Healthcare Spending, by Region (Government, Commercial, Private, Out-of-Pocket), 2023
6. Competitive Landscape
6.1 List of Major Companies, By Region
6.2 Market Share Analysis, By Region
6.3 Product Benchmarking
6.3.1 Product specifications and features
6.3.2 Pricing
6.4 Strategic Initiatives
6.4.1 Marketing and promotional activities
6.4.2 Distribution and Supply Chain Strategies
6.4.3 Expansion plans and new Product launches
6.4.4 Strategic partnerships and collaborations
6.5 Technological Advancements
6.6 Market Positioning and Branding
7. 3D Printing Medical Devices Market Segmentation, by Component
7.1 Chapter Overview
7.2 Software and Services
7.2.1 Software and Services Market Trends Analysis (2020-2032)
7.2.2 Software and Services Market Size Estimates and Forecasts to 2032 (USD Billion)
7.3 Equipment
7.3.1 Equipment Market Trends Analysis (2020-2032)
7.3.2 Equipment Market Size Estimates and Forecasts to 2032 (USD Billion)
7.4 3D Printers
7.4.1 3D Printers Market Trends Analysis (2020-2032)
7.4.2 3D Printers Market Size Estimates and Forecasts to 2032 (USD Billion)
7.5 3D Bioprinters
7.5.1 3D Bioprinters Market Trends Analysis (2020-2032)
7.5.2 3D Bioprinters Market Size Estimates and Forecasts to 2032 (USD Billion)
7.6 Biomaterials
7.6.1 Biomaterials Market Trends Analysis (2020-2032)
7.6.2 Biomaterials Market Size Estimates and Forecasts to 2032 (USD Billion)
8. 3D Printing Medical Devices Market Segmentation, by Type
8.1 Chapter Overview
8.2 Surgical Guides
8.2.1 Surgical Guides Market Trends Analysis (2020-2032)
8.2.2 Surgical Guides Market Size Estimates and Forecasts to 2032 (USD Billion)
8.3 Dental Guides
8.3.1 Dental Guides Market Trends Analysis (2020-2032)
8.3.2 Dental Guides Market Size Estimates and Forecasts to 2032 (USD Billion)
8.4 Craniomaxillofacial Guides
8.4.1 Craniomaxillofacial Guides Market Trends Analysis (2020-2032)
8.4.2 Craniomaxillofacial Guides Market Size Estimates and Forecasts to 2032 (USD Billion)
8.5 Orthopedic Guides
8.5.1 Orthopedic Guides Market Trends Analysis (2020-2032)
8.5.2 Orthopedic Guides Market Size Estimates and Forecasts to 2032 (USD Billion)
8.6 Surgical Instruments
8.6.1 Surgical Instruments Market Trends Analysis (2020-2032)
8.6.2 Surgical Instruments Market Size Estimates and Forecasts to 2032 (USD Billion)
8.7 Retractors
8.7.1 Retractors Market Trends Analysis (2020-2032)
8.7.2 Retractors Market Size Estimates and Forecasts to 2032 (USD Billion)
9. 3D Printing Medical Devices Market Segmentation, by Technology
9.1 Chapter Overview
9.2 Electron Beam Melting (EBM) Technology
9.2.1 Electron Beam Melting (EBM) Technology Market Trends Analysis (2020-2032)
9.2.2 Electron Beam Melting (EBM) Technology Market Size Estimates and Forecasts to 2032 (USD Billion)
9.3 Laser Beam Melting (LBM) Technology
9.3.1 Laser Beam Melting (LBM) Technology Market Trends Analysis (2020-2032)
9.3.2 Laser Beam Melting (LBM) Technology Market Size Estimates and Forecasts to 2032 (USD Billion)
9.4 Direct Metal Laser Sintering (DMLS)
9.4.1 Direct Metal Laser Sintering (DMLS) Market Trends Analysis (2020-2032)
9.4.2 Direct Metal Laser Sintering (DMLS) Market Size Estimates and Forecasts to 2032 (USD Billion)
9.5 Selective Laser Melting (SLM)
9.5.1 Selective Laser Melting (SLM) Market Trends Analysis (2020-2032)
9.5.2 Selective Laser Melting (SLM) Market Size Estimates and Forecasts to 2032 (USD Billion)
9.6 Selective Laser Sintering (SLS)
9.6.1 Selective Laser Sintering (SLS) Market Trends Analysis (2020-2032)
9.6.2 Selective Laser Sintering (SLS)Selective Laser Sintering (SLS) Market Size Estimates and Forecasts to 2032 (USD Billion)
9.7 Photopolymerization
9.7.1 Photopolymerization Market Trends Analysis (2020-2032)
9.7.2 Photopolymerization Market Size Estimates and Forecasts to 2032 (USD Billion)
10. Regional Analysis
10.1 Chapter Overview
10.2 North America
10.2.1 Trends Analysis
10.2.2 North America 3D Printing Medical Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.2.3 North America 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.2.4 North America 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.2.5 North America 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.2.6 USA
10.2.6.1 USA 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.2.6.2 USA 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.2.6.3 USA 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.2.7 Canada
10.2.7.1 Canada 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.2.7.2 Canada 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.2.7.3 Canada 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.2.8 Mexico
10.2.8.1 Mexico 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.2.8.2 Mexico 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.2.8.3 Mexico 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.3 Europe
10.3.1 Eastern Europe
10.3.1.1 Trends Analysis
10.3.1.2 Eastern Europe 3D Printing Medical Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.3.1.3 Eastern Europe 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.3.1.4 Eastern Europe 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.1.5 Eastern Europe 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.3.1.6 Poland
10.3.1.6.1 Poland 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.3.1.6.2 Poland 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.1.6.3 Poland 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.3.1.7 Romania
10.3.1.7.1 Romania 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.3.1.7.2 Romania 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.1.7.3 Romania 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.3.1.8 Hungary
10.3.1.8.1 Hungary 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.3.1.8.2 Hungary 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.1.8.3 Hungary 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.3.1.9 turkey
10.3.1.9.1 Turkey 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.3.1.9.2 Turkey 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.1.9.3 Turkey 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.3.1.10 Rest of Eastern Europe
10.3.1.10.1 Rest of Eastern Europe 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.3.1.10.2 Rest of Eastern Europe 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.1.10.3 Rest of Eastern Europe 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.3.2 Western Europe
10.3.2.1 Trends Analysis
10.3.2.2 Western Europe 3D Printing Medical Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.3.2.3 Western Europe 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.3.2.4 Western Europe 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.2.5 Western Europe 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.3.2.6 Germany
10.3.2.6.1 Germany 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.3.2.6.2 Germany 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.2.6.3 Germany 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.3.2.7 France
10.3.2.7.1 France 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.3.2.7.2 France 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.2.7.3 France 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.3.2.8 UK
10.3.2.8.1 UK 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.3.2.8.2 UK 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.2.8.3 UK 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.3.2.9 Italy
10.3.2.9.1 Italy 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.3.2.9.2 Italy 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.2.9.3 Italy 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.3.2.10 Spain
10.3.2.10.1 Spain 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.3.2.10.2 Spain 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.2.10.3 Spain 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.3.2.11 Netherlands
10.3.2.11.1 Netherlands 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.3.2.11.2 Netherlands 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.2.11.3 Netherlands 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.3.2.12 Switzerland
10.3.2.12.1 Switzerland 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.3.2.12.2 Switzerland 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.2.12.3 Switzerland 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.3.2.13 Austria
10.3.2.13.1 Austria 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.3.2.13.2 Austria 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.2.13.3 Austria 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.3.2.14 Rest of Western Europe
10.3.2.14.1 Rest of Western Europe 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.3.2.14.2 Rest of Western Europe 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.3.2.14.3 Rest of Western Europe 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.4 Asia Pacific
10.4.1 Trends Analysis
10.4.2 Asia Pacific 3D Printing Medical Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.4.3 Asia Pacific 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.4.4 Asia Pacific 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.4.5 Asia Pacific 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.4.6 China
10.4.6.1 China 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.4.6.2 China 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.4.6.3 China 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.4.7 India
10.4.7.1 India 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.4.7.2 India 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.4.7.3 India 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.4.8 Japan
10.4.8.1 Japan 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.4.8.2 Japan 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.4.8.3 Japan 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.4.9 South Korea
10.4.9.1 South Korea 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.4.9.2 South Korea 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.4.9.3 South Korea 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.4.10 Vietnam
10.4.10.1 Vietnam 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.4.10.2 Vietnam 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.4.10.3 Vietnam 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.4.11 Singapore
10.4.11.1 Singapore 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.4.11.2 Singapore 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.4.11.3 Singapore 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.4.12 Australia
10.4.12.1 Australia 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.4.12.2 Australia 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.4.12.3 Australia 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.4.13 Rest of Asia Pacific
10.4.13.1 Rest of Asia Pacific 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.4.13.2 Rest of Asia Pacific 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.4.13.3 Rest of Asia Pacific 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.5 Middle East and Africa
10.5.1 Middle East
10.5.1.1 Trends Analysis
10.5.1.2 Middle East 3D Printing Medical Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.5.1.3 Middle East 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.5.1.4 Middle East 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.5.1.5 Middle East 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.5.1.6 UAE
10.5.1.6.1 UAE 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.5.1.6.2 UAE 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.5.1.6.3 UAE 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.5.1.7 Egypt
10.5.1.7.1 Egypt 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.5.1.7.2 Egypt 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.5.1.7.3 Egypt 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.5.1.8 Saudi Arabia
10.5.1.8.1 Saudi Arabia 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.5.1.8.2 Saudi Arabia 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.5.1.8.3 Saudi Arabia 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.5.1.9 Qatar
10.5.1.9.1 Qatar 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.5.1.9.2 Qatar 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.5.1.9.3 Qatar 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.5.1.10 Rest of Middle East
10.5.1.10.1 Rest of Middle East 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.5.1.10.2 Rest of Middle East 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.5.1.10.3 Rest of Middle East 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.5.2 Africa
10.5.2.1 Trends Analysis
10.5.2.2 Africa 3D Printing Medical Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.5.2.3 Africa 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.5.2.4 Africa 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.5.2.5 Africa 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.5.2.6 South Africa
10.5.2.6.1 South Africa 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.5.2.6.2 South Africa 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.5.2.6.3 South Africa 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.5.2.7 Nigeria
10.5.2.7.1 Nigeria 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.5.2.7.2 Nigeria 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.5.2.7.3 Nigeria 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.5.2.8 Rest of Africa
10.5.2.8.1 Rest of Africa 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.5.2.8.2 Rest of Africa 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.5.2.8.3 Rest of Africa 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.6 Latin America
10.6.1 Trends Analysis
10.6.2 Latin America 3D Printing Medical Devices Market Estimates and Forecasts, by Country (2020-2032) (USD Billion)
10.6.3 Latin America 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.6.4 Latin America 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.6.5 Latin America 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.6.6 Brazil
10.6.6.1 Brazil 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.6.6.2 Brazil 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.6.6.3 Brazil 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.6.7 Argentina
10.6.7.1 Argentina 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.6.7.2 Argentina 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.6.7.3 Argentina 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.6.8 Colombia
10.6.8.1 Colombia 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.6.8.2 Colombia 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.6.8.3 Colombia 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
10.6.9 Rest of Latin America
10.6.9.1 Rest of Latin America 3D Printing Medical Devices Market Estimates and Forecasts, by Component (2020-2032) (USD Billion)
10.6.9.2 Rest of Latin America 3D Printing Medical Devices Market Estimates and Forecasts, by Type (2020-2032) (USD Billion)
10.6.9.3 Rest of Latin America 3D Printing Medical Devices Market Estimates and Forecasts, by Technology (2020-2032) (USD Billion)
11. Company Profiles
11.1 Stratasys Ltd.
11.1.1 Company Overview
11.1.2 Financial
11.1.3 Product Types/ Services Offered
11.1.4 SWOT Analysis
11.2 EnvisionTEC
11.2.1 Company Overview
11.2.2 Financial
11.2.3 Product Types/ Services Offered
11.2.4 SWOT Analysis
11.3 3D Systems, Inc.
11.3.1 Company Overview
11.3.2 Financial
11.3.3 Product Types/ Services Offered
11.3.4 SWOT Analysis
11.4 EOS GmbH
11.4.1 Company Overview
11.4.2 Financial
11.4.3 Product Types/ Services Offered
11.4.4 SWOT Analysis
11.5 Renishaw plc
11.5.1 Company Overview
11.5.2 Financial
11.5.3 Product Types/ Services Offered
11.5.4 SWOT Analysis
11.6 Desktop Metal, Inc.
11.6.1 Company Overview
11.6.2 Financial
11.6.3 Product Types/ Services Offered
11.6.4 SWOT Analysis
11.7 3T Additive Manufacturing Ltd.
11.7.1 Company Overview
11.7.2 Financial
11.7.3 Product Types/ Services Offered
11.7.4 SWOT Analysis
11.8 General Electric Company
11.8.1 Company Overview
11.8.2 Financial
11.8.3 Product Types/ Services Offered
11.8.4 SWOT Analysis
11.9 Organovo Holdings Inc.
11.9.1 Company Overview
11.9.2 Financial
11.9.3 Product Types/ Services Offered
11.9.4 SWOT Analysis
11.10 Dentsply Sirona
11.10.1 Company Overview
11.10.2 Financial
11.10.3 Product Types/ Services Offered
11.10.4 SWOT Analysis
12. Use Cases and Best Practices
13. Conclusion
An accurate research report requires proper strategizing as well as implementation. There are multiple factors involved in the completion of good and accurate research report and selecting the best methodology to compete the research is the toughest part. Since the research reports we provide play a crucial role in any company’s decision-making process, therefore we at SNS Insider always believe that we should choose the best method which gives us results closer to reality. This allows us to reach at a stage wherein we can provide our clients best and accurate investment to output ratio.
Each report that we prepare takes a timeframe of 350-400 business hours for production. Starting from the selection of titles through a couple of in-depth brain storming session to the final QC process before uploading our titles on our website we dedicate around 350 working hours. The titles are selected based on their current market cap and the foreseen CAGR and growth.
The 5 steps process:
Step 1: Secondary Research:
Secondary Research or Desk Research is as the name suggests is a research process wherein, we collect data through the readily available information. In this process we use various paid and unpaid databases which our team has access to and gather data through the same. This includes examining of listed companies’ annual reports, Journals, SEC filling etc. Apart from this our team has access to various associations across the globe across different industries. Lastly, we have exchange relationships with various university as well as individual libraries.

Step 2: Primary Research
When we talk about primary research, it is a type of study in which the researchers collect relevant data samples directly, rather than relying on previously collected data. This type of research is focused on gaining content specific facts that can be sued to solve specific problems. Since the collected data is fresh and first hand therefore it makes the study more accurate and genuine.
We at SNS Insider have divided Primary Research into 2 parts.
Part 1 wherein we interview the KOLs of major players as well as the upcoming ones across various geographic regions. This allows us to have their view over the market scenario and acts as an important tool to come closer to the accurate market numbers. As many as 45 paid and unpaid primary interviews are taken from both the demand and supply side of the industry to make sure we land at an accurate judgement and analysis of the market.
This step involves the triangulation of data wherein our team analyses the interview transcripts, online survey responses and observation of on filed participants. The below mentioned chart should give a better understanding of the part 1 of the primary interview.

Part 2: In this part of primary research the data collected via secondary research and the part 1 of the primary research is validated with the interviews from individual consultants and subject matter experts.
Consultants are those set of people who have at least 12 years of experience and expertise within the industry whereas Subject Matter Experts are those with at least 15 years of experience behind their back within the same space. The data with the help of two main processes i.e., FGDs (Focused Group Discussions) and IDs (Individual Discussions). This gives us a 3rd party nonbiased primary view of the market scenario making it a more dependable one while collation of the data pointers.
Step 3: Data Bank Validation
Once all the information is collected via primary and secondary sources, we run that information for data validation. At our intelligence centre our research heads track a lot of information related to the market which includes the quarterly reports, the daily stock prices, and other relevant information. Our data bank server gets updated every fortnight and that is how the information which we collected using our primary and secondary information is revalidated in real time.

Step 4: QA/QC Process
After all the data collection and validation our team does a final level of quality check and quality assurance to get rid of any unwanted or undesired mistakes. This might include but not limited to getting rid of the any typos, duplication of numbers or missing of any important information. The people involved in this process include technical content writers, research heads and graphics people. Once this process is completed the title gets uploader on our platform for our clients to read it.
Step 5: Final QC/QA Process:
This is the last process and comes when the client has ordered the study. In this process a final QA/QC is done before the study is emailed to the client. Since we believe in giving our clients a good experience of our research studies, therefore, to make sure that we do not lack at our end in any way humanly possible we do a final round of quality check and then dispatch the study to the client.
By Component
Software and Services
Equipment
3D Printers
3D Bioprinters
Biomaterials
By Type
Surgical Guides
Dental Guides
Craniomaxillofacial Guides
Orthopedic Guides
Surgical Instruments
Retractors
By Technology
Electron Beam Melting (EBM) Technology
Laser Beam Melting (LBM) Technology
Direct Metal Laser Sintering (DMLS)
Selective Laser Melting (SLM)
Selective Laser Sintering (SLS)
Photopolymerization
Request for Segment Customization as per your Business Requirement: Segment Customization Request
REGIONAL COVERAGE:
North America
US
Canada
Mexico
Europe
Eastern Europe
Poland
Romania
Hungary
Turkey
Rest of Eastern Europe
Western Europe
Germany
France
UK
Italy
Spain
Netherlands
Switzerland
Austria
Rest of Western Europe
Asia Pacific
China
India
Japan
South Korea
Vietnam
Singapore
Australia
Rest of Asia Pacific
Middle East & Africa
Middle East
UAE
Egypt
Saudi Arabia
Qatar
Rest of the Middle East
Africa
Nigeria
South Africa
Rest of Africa
Latin America
Brazil
Argentina
Colombia
Request for Country Level Research Report: Country Level Customization Request
Available Customization
With the given market data, SNS Insider offers customization as per the company’s specific needs. The following customization options are available for the report:
Product Analysis
Criss-Cross segment analysis (e.g. Product X Application)
Product Matrix which gives a detailed comparison of the product portfolio of each company
Geographic Analysis
Additional countries in any of the regions
Company Information
Detailed analysis and profiling of additional market players (Up to five)
The ECG Patch & Holter Monitor Market size was valued at USD 1.60 Billion in 2023 and is expected to reach USD 7.78 Billion by 2032 and grow at a CAGR of 19.23% over the forecast period 2024-2032.
The Surgical Procedures Market Size was valued at USD 108.27 Million in 2023 and is expected to reach USD 173.73 Million by 2032 and grow at a CAGR of 5.61% over the forecast period 2024-2032.
The Elderly Walker Market was valued at USD 1.56 billion in 2023 and is expected to reach USD 2.73 billion by 2032, growing at a CAGR of 6.44% from 2024 to 2032
The Metagenomics Market Size was valued at USD 2.11 Billion in 2023 and is expected to reach USD 6.70 Billion by 2032, growing at a CAGR of 13.69% over the forecast period of 2024-2032.
The Podiatry Services Market was valued at USD 4.61 billion in 2023 and is expected to reach USD 5.81 billion by 2032, growing at a CAGR of 2.65% from 2024-2032.
The Empty IV Bags Market size was valued at USD 4.44 Billion in 2023 and is expected to reach USD 8.86 Billion by 2032 and grow at a CAGR of 7.31% over the forecast period 2024-2032.
Hi! Click one of our member below to chat on Phone